444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The global DNA vaccines market is witnessing significant growth due to advancements in biotechnology and the increasing prevalence of infectious diseases. DNA vaccines are a type of genetic vaccine that use a small, circular DNA molecule to deliver genetic material into cells, triggering an immune response. These vaccines offer several advantages over traditional vaccines, such as improved safety, ease of production, and the potential for rapid development.
Meaning
DNA vaccines are a novel approach to vaccination that utilize the body’s own cellular machinery to produce an immune response. Unlike traditional vaccines, which often use weakened or inactivated forms of pathogens, DNA vaccines introduce a small piece of the pathogen’s DNA into cells. This DNA encodes for specific antigens, which are proteins that stimulate an immune response. Once the DNA is taken up by cells, it instructs them to produce these antigens, triggering the immune system to recognize and respond to them.
Executive Summary
The global DNA vaccines market is poised for substantial growth in the coming years, driven by factors such as increasing investments in research and development, rising prevalence of infectious diseases, and technological advancements in the field of genetic engineering. The market is highly competitive, with several key players vying for market share. However, challenges such as regulatory hurdles and public skepticism towards genetic vaccines may impede market growth to some extent.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
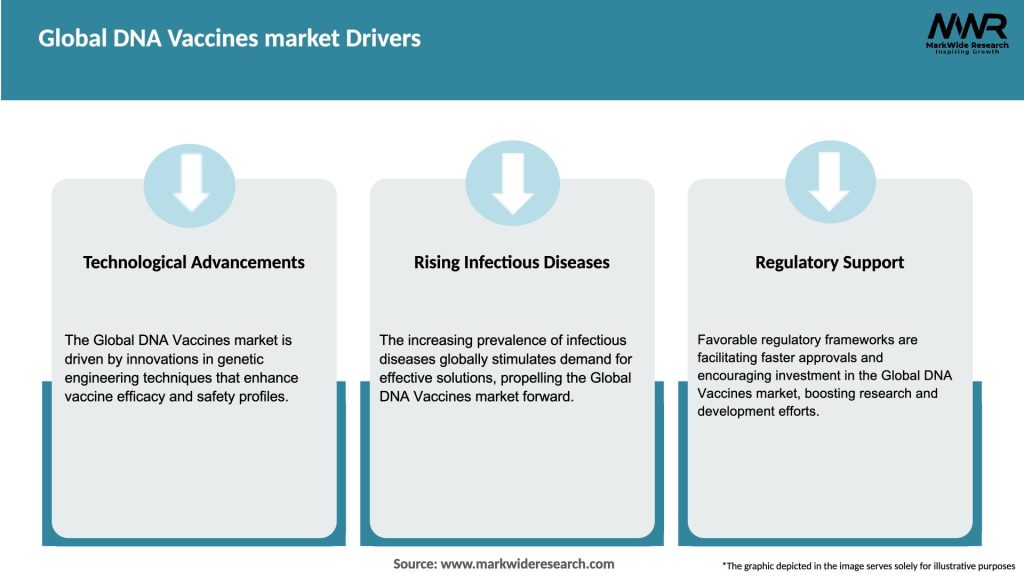
Market Drivers
Several key factors are driving the growth of the global DNA vaccines market:
Market Restraints
Despite the positive growth prospects, the global DNA vaccines market faces certain challenges:
Market Opportunities
The global DNA vaccines market presents several opportunities for growth and innovation:

Market Dynamics
The global DNA vaccines market is characterized by intense competition and ongoing research and development activities. Market dynamics are influenced by various factors, including technological advancements, government regulations, funding initiatives, and collaborations between key market players. Continuous innovation and the ability to address unmet medical needs will be crucial for sustained market growth.
Regional Analysis
The global DNA vaccines market can be segmented into several regions, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America currently dominates the market, driven by the presence of established pharmaceutical companies, robust healthcare infrastructure, and significant investments in research and development. However, Asia Pacific is expected to witness rapid growth due to increasing healthcare expenditure, rising disease burden, and a growing focus on vaccine development.
Competitive Landscape
Leading Companies in the Global DNA Vaccines Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The global DNA vaccines market can be segmented based on various factors, including:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The global DNA vaccines market offers several benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats associated with the global DNA vaccines market:
Market Key Trends
The global DNA vaccines market is influenced by several key trends:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the DNA vaccines market. The urgent need for effective vaccines to combat the virus has accelerated research and development efforts in genetic vaccines, including DNA vaccines. Several DNA-based COVID-19 vaccines, such as those developed by Pfizer-BioNTech and Moderna, have shown high efficacy in clinical trials and have been authorized for emergency use.
The successful development and deployment of DNA vaccines for COVID-19 have bolstered confidence in this technology and demonstrated its potential in addressing infectious diseases. The pandemic has also highlighted the need for rapid vaccine development and manufacturing capabilities, which DNA vaccines can provide.
However, the COVID-19 pandemic has also exposed challenges in vaccine distribution, cold chain logistics, and vaccine hesitancy. Overcoming these challenges is crucial for the widespread adoption and successful implementation of DNA vaccines in pandemic control and future disease outbreaks.
Key Industry Developments
Several key industry developments have shaped the global DNA vaccines market:
Analyst Suggestions
Based on market trends and dynamics, analysts suggest the following strategies for industry participants:
Future Outlook
The future outlook for the global DNA vaccines market is highly promising. Advancements in genetic engineering, growing acceptance of genetic vaccines, and the lessons learned from the COVID-19 pandemic are expected to drive market growth. The market is likely to witness increased investment in research and development, expansion into emerging markets, and the development of innovative delivery systems.
The application of DNA vaccines in preventive and therapeutic settings is anticipated to expand, with a focus on addressing infectious diseases, cancer, and genetic disorders. Personalized medicine approaches and the integration of DNA vaccines with gene therapies hold significant potential for the future.
However, challenges such as regulatory complexities, public skepticism, and cold chain requirements need to be addressed to ensure the successful implementation and widespread adoption of DNA vaccines. Overcoming these challenges will require collaborative efforts, continued research, and targeted strategies to build public trust and streamline regulatory processes.
Conclusion
The global DNA vaccines market is experiencing significant growth, driven by advancements in genetic engineering, increasing prevalence of infectious diseases, and the potential for rapid vaccine development. DNA vaccines offer advantages such as improved safety, simplified manufacturing processes, and the potential for broad-spectrum protection.
While the market presents opportunities for industry participants, challenges such as regulatory complexities and public skepticism need to be addressed. Collaborations, diversification, and investments in research and development are crucial for staying competitive in this rapidly evolving market.
What is DNA Vaccines?
DNA vaccines are a type of genetic vaccine that use genetically engineered DNA to induce an immune response against specific pathogens. They are designed to provide protection against infectious diseases and have potential applications in cancer therapy.
What are the key players in the Global DNA Vaccines market?
Key players in the Global DNA Vaccines market include Inovio Pharmaceuticals, Zydus Cadila, and Merck & Co., among others. These companies are involved in the development and commercialization of innovative DNA vaccine technologies.
What are the drivers of growth in the Global DNA Vaccines market?
The growth of the Global DNA Vaccines market is driven by increasing incidences of infectious diseases, advancements in genetic engineering technologies, and rising investments in vaccine research and development. Additionally, the growing focus on personalized medicine is contributing to market expansion.
What challenges does the Global DNA Vaccines market face?
The Global DNA Vaccines market faces challenges such as regulatory hurdles, public skepticism regarding vaccine safety, and the complexity of manufacturing DNA vaccines. These factors can hinder the timely development and approval of new vaccines.
What opportunities exist in the Global DNA Vaccines market?
Opportunities in the Global DNA Vaccines market include the potential for developing vaccines for emerging infectious diseases and the application of DNA vaccines in cancer immunotherapy. The increasing collaboration between biotech firms and research institutions also presents growth prospects.
What trends are shaping the Global DNA Vaccines market?
Trends shaping the Global DNA Vaccines market include the integration of novel delivery systems, such as electroporation, and the exploration of combination therapies. Additionally, there is a growing emphasis on rapid response vaccine development in light of global health emergencies.
Global DNA Vaccines market
| Segmentation Details | Description |
|---|---|
| Product Type | Plasmid DNA Vaccines, Viral Vector Vaccines, RNA Vaccines, Protein Subunit Vaccines |
| End User | Pharmaceutical Companies, Research Institutions, Biotechnology Firms, Government Agencies |
| Delivery Mode | Intramuscular, Intradermal, Oral, Nasal |
| Application | Infectious Diseases, Cancer Immunotherapy, Autoimmune Disorders, Veterinary Medicine |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at