444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The global cystic fibrosis diagnostic tests market is experiencing steady growth due to several factors, including increasing awareness about the disease, advancements in diagnostic technologies, and rising prevalence of cystic fibrosis worldwide. Cystic fibrosis is a genetic disorder that affects the respiratory and digestive systems, leading to the production of thick and sticky mucus. Diagnostic tests play a crucial role in early detection and management of the disease, allowing for timely intervention and improved patient outcomes.
Cystic fibrosis is a hereditary disease caused by mutations in the CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene. These mutations affect the production and function of the CFTR protein, resulting in the accumulation of thick and sticky mucus in various organs, particularly the lungs and digestive system. Cystic fibrosis diagnostic tests are used to identify these mutations, assess lung function, and monitor disease progression.
Executive Summary
The global cystic fibrosis diagnostic tests market is expected to witness substantial growth in the coming years. This growth can be attributed to factors such as increasing awareness about cystic fibrosis, technological advancements in diagnostic tests, and the rising prevalence of the disease. The market is characterized by the presence of several key players offering a wide range of diagnostic tests and services. North America holds a significant market share, followed by Europe and Asia Pacific. However, emerging economies in Asia Pacific are projected to offer lucrative opportunities for market expansion.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
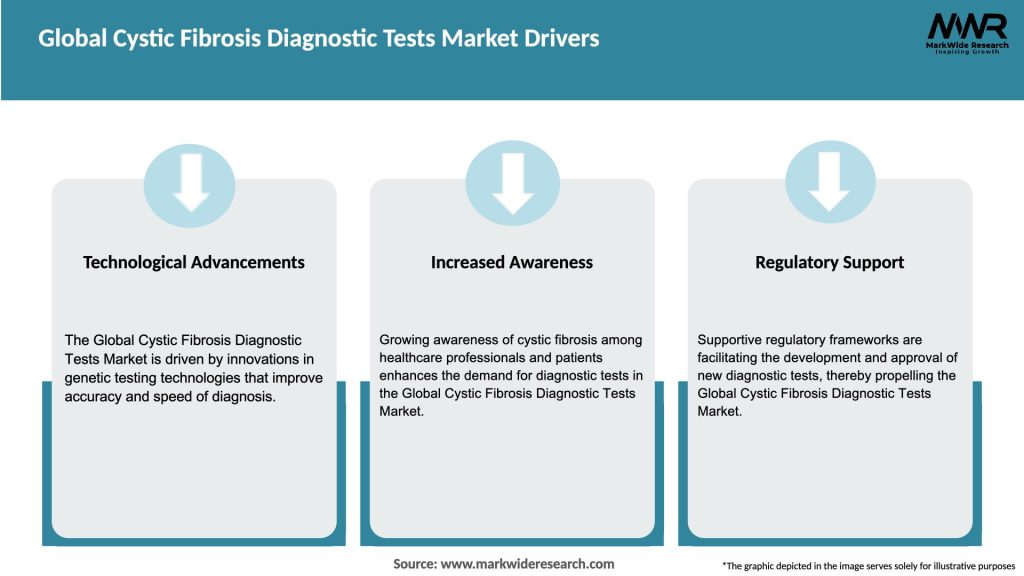
Market Drivers
Several key drivers contribute to the growth of the global cystic fibrosis diagnostic tests market:
Market Restraints
Despite the positive market outlook, certain factors may impede the growth of the cystic fibrosis diagnostic tests market:
Market Opportunities
The global cystic fibrosis diagnostic tests market presents several opportunities for growth and expansion:

Market Dynamics
The global cystic fibrosis diagnostic tests market is driven by a combination of factors, including increasing disease prevalence, technological advancements, and supportive government initiatives. These dynamics create a favorable environment for market growth, leading to the development of innovative diagnostic solutions and expansion into untapped markets. However, challenges such as high costs, regulatory requirements, and limited access to diagnostic facilities can hinder market growth. It is essential for key stakeholders to collaborate and address these challenges while capitalizing on emerging opportunities.
Regional Analysis
The global cystic fibrosis diagnostic tests market can be analyzed based on key regions, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
Competitive Landscape
Leading Companies in the Global Cystic Fibrosis Diagnostic Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The global cystic fibrosis diagnostic tests market can be segmented based on:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the cystic fibrosis diagnostic tests market. While the primary focus of healthcare systems has been on managing the pandemic, the diagnosis and management of other chronic diseases, including cystic fibrosis, have been affected. Access to diagnostic facilities, routine screenings, and follow-up visits may have been disrupted due to lockdowns and overwhelmed healthcare systems.
However, the pandemic has also highlighted the importance of early diagnosis and effective disease management. The need for improved diagnostic tests, including rapid and accurate point-of-care tests, has become even more apparent. The development of innovative testing solutions and the integration of telemedicine and remote monitoring technologies have gained momentum during this time.
Despite the challenges posed by the pandemic, the cystic fibrosis diagnostic tests market is expected to recover and witness steady growth in the post-pandemic period. Continued efforts to raise awareness, improve access to diagnostic services, and focus on personalized and targeted therapies will drive market growth.
Key Industry Developments
Analyst Suggestions
Future Outlook
The global cystic fibrosis diagnostic tests market is expected to witness significant growth in the coming years. Increasing disease prevalence, advancements in diagnostic technologies, and rising awareness about cystic fibrosis will drive market expansion. The development of non-invasive, rapid, and accurate diagnostic tests will improve early detection and facilitate timely intervention. Collaboration between pharmaceutical companies, diagnostic laboratories, and research institutions will lead to the development of innovative diagnostic solutions. Continued investment in research and development activities and expansion into emerging economies will unlock new opportunities for market players. Overall, the future outlook for the cystic fibrosis diagnostic tests market remains optimistic.
Conclusion
The global cystic fibrosis diagnostic tests market is experiencing steady growth due to increasing disease prevalence, technological advancements, and rising awareness. Diagnostic tests play a crucial role in early detection, disease management, and improved patient outcomes. The market is driven by factors such as increasing disease awareness, supportive government initiatives, and collaborations between pharmaceutical companies and diagnostic laboratories.
However, challenges related to cost, regulatory requirements, and limited access to diagnostic facilities exist. Key industry developments, including the introduction of innovative diagnostic solutions, personalized medicine approaches, and integration of digital health technologies, will shape the market’s future. The market is poised for expansion, particularly in emerging economies with a high burden of cystic fibrosis. Continued investment in research and development activities, collaborations, and awareness initiatives will contribute to the growth and advancement of the cystic fibrosis diagnostic tests market.
What is Cystic Fibrosis Diagnostic Tests?
Cystic Fibrosis Diagnostic Tests are medical assessments used to identify cystic fibrosis, a genetic disorder that affects the lungs and digestive system. These tests typically include sweat tests, genetic testing, and newborn screening to detect the presence of the disease early.
What are the key players in the Global Cystic Fibrosis Diagnostic Tests Market?
Key players in the Global Cystic Fibrosis Diagnostic Tests Market include companies like Thermo Fisher Scientific, Roche Diagnostics, and Siemens Healthineers, which are known for their innovative diagnostic solutions and technologies, among others.
What are the drivers of growth in the Global Cystic Fibrosis Diagnostic Tests Market?
The growth of the Global Cystic Fibrosis Diagnostic Tests Market is driven by increasing awareness of cystic fibrosis, advancements in genetic testing technologies, and the rising prevalence of the disease. Additionally, government initiatives for early diagnosis and treatment are contributing to market expansion.
What challenges does the Global Cystic Fibrosis Diagnostic Tests Market face?
The Global Cystic Fibrosis Diagnostic Tests Market faces challenges such as high costs associated with advanced diagnostic tests and the need for skilled professionals to conduct these tests. Furthermore, variations in regulatory standards across regions can hinder market growth.
What opportunities exist in the Global Cystic Fibrosis Diagnostic Tests Market?
Opportunities in the Global Cystic Fibrosis Diagnostic Tests Market include the development of more cost-effective testing methods and the integration of digital health technologies. Additionally, increasing collaborations between research institutions and diagnostic companies can enhance innovation in testing.
What trends are shaping the Global Cystic Fibrosis Diagnostic Tests Market?
Trends shaping the Global Cystic Fibrosis Diagnostic Tests Market include the rise of personalized medicine, where treatments are tailored to individual genetic profiles, and the growing use of telemedicine for remote consultations and follow-ups. Moreover, advancements in point-of-care testing are making diagnostics more accessible.
Global Cystic Fibrosis Diagnostic Tests Market:
| Segmentation Details | Description |
|---|---|
| Test Type | Sweat Test, Genetic Testing, Imaging Tests, Lung Function Tests, Others |
| Age Group | Pediatric, Adult |
| End-user | Hospitals, Diagnostic Laboratories, Clinics, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Global Cystic Fibrosis Diagnostic Tests Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at