444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The Global CRO (Contract Research Organization) Services market is a rapidly growing industry that plays a crucial role in supporting the development and commercialization of pharmaceutical, biotechnology, and medical device products. CROs offer a range of services to assist companies in various stages of the research and development (R&D) process, including clinical trials, regulatory compliance, data management, and post-marketing surveillance.
CRO Services refer to outsourced research and development activities provided by specialized organizations. These organizations collaborate with pharmaceutical, biotechnology, and medical device companies to streamline the drug development process and ensure regulatory compliance. By leveraging the expertise and infrastructure of CROs, companies can reduce costs, accelerate timelines, and mitigate risks associated with R&D.
Executive Summary
The Global CRO Services market has witnessed significant growth over the past decade, driven by factors such as increasing R&D expenditure, the complexity of clinical trials, and the growing demand for specialized expertise. The market is highly competitive, with both global and regional players vying for market share. Key market participants include large CROs with global reach as well as niche players specializing in specific therapeutic areas or service offerings.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
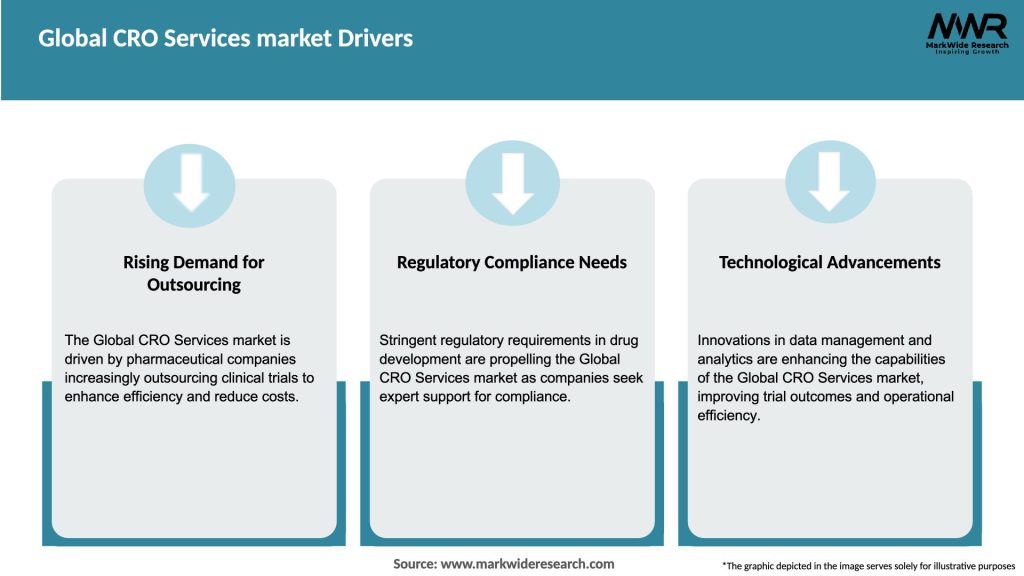
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Global CRO Services market is highly dynamic and influenced by various factors, including technological advancements, regulatory changes, and market consolidation. The market is characterized by intense competition, with CROs constantly striving to differentiate themselves by offering innovative services, niche expertise, and a global presence. Collaboration and partnerships between CROs and pharmaceutical companies are also on the rise, fostering synergy and driving market growth.
Regional Analysis
The Global CRO Services market is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America dominates the market, primarily driven by the presence of major pharmaceutical companies, robust R&D infrastructure, and favorable regulatory policies. Europe is the second-largest market, with increasing investments in clinical research and the presence of renowned CROs. The Asia Pacific region is experiencing significant growth, fueled by the rising outsourcing trend, a large patient population, and improving healthcare infrastructure.
Competitive Landscape
Leading Companies in the Global CRO Services Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
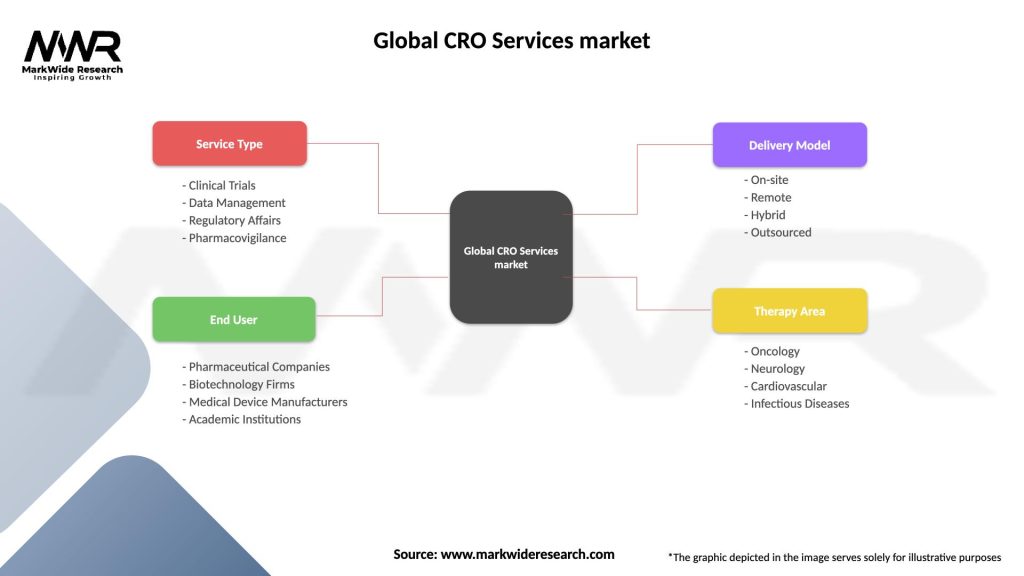
Segmentation
The Global CRO Services market can be segmented based on service type, therapeutic area, end-user, and geography. Service types include clinical trial management, regulatory services, data management, biostatistics, medical writing, and others. Therapeutic areas encompass oncology, cardiovascular diseases, infectious diseases, neurology, and others. End-users of CRO services include pharmaceutical and biotechnology companies, medical device manufacturers, academic and research institutions, and contract manufacturing organizations (CMOs).
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Global CRO Services market. The suspension of clinical trials, disruption of supply chains, and the need for accelerated research on vaccines and therapeutics have posed challenges and opportunities for CROs. While some trials were delayed or put on hold, there has been an increased demand for CRO services in COVID-19-related research, including clinical trials, data management, and regulatory support.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Global CRO Services market is expected to continue its growth trajectory in the coming years. Factors such as increasing R&D expenditure, the complexity of clinical trials, and the need for specialized expertise will drive market growth. The adoption of advanced technologies, expansion into emerging markets, and the development of personalized therapies will present new opportunities for CROs. However, challenges related to regulatory compliance, quality control, and competition will need to be effectively addressed to sustain growth.
Conclusion
The Global CRO Services market plays a critical role in supporting the research and development activities of pharmaceutical, biotechnology, and medical device companies. The market is driven by factors such as increasing R&D expenditure, the complexity of clinical trials, and the demand for specialized expertise. CROs offer a wide range of services, including clinical trial management, regulatory support, data management, and biostatistics. The market is highly competitive, with key players focusing on expanding their service offerings, strengthening their global presence, and fostering strategic collaborations. The future outlook for the market remains positive, with opportunities arising from emerging markets, advanced technologies, and the development of targeted therapies. CROs will need to adapt to regulatory changes, embrace digital transformation, and differentiate themselves through niche expertise to thrive in this dynamic industry.
What is CRO Services?
CRO Services, or Contract Research Organization Services, refer to a range of outsourced research services provided to the pharmaceutical, biotechnology, and medical device industries. These services include clinical trial management, data management, regulatory affairs, and biostatistics, among others.
What are the key players in the Global CRO Services market?
Key players in the Global CRO Services market include Covance, Parexel, and ICON plc, which provide comprehensive research and development services. These companies are known for their expertise in clinical trials and regulatory compliance, among others.
What are the main drivers of growth in the Global CRO Services market?
The main drivers of growth in the Global CRO Services market include the increasing demand for outsourcing clinical trials, the rise in R&D expenditure by pharmaceutical companies, and the need for faster drug development processes. Additionally, advancements in technology and data analytics are also contributing to market expansion.
What challenges does the Global CRO Services market face?
The Global CRO Services market faces challenges such as regulatory complexities, data privacy concerns, and the need for skilled professionals. These factors can hinder the efficiency and effectiveness of clinical trials and research services.
What opportunities exist in the Global CRO Services market?
Opportunities in the Global CRO Services market include the growing trend of personalized medicine, the expansion of clinical trials in emerging markets, and the integration of artificial intelligence in research processes. These factors are expected to enhance service offerings and improve patient outcomes.
What trends are shaping the Global CRO Services market?
Trends shaping the Global CRO Services market include the increasing use of digital technologies in clinical trials, the rise of patient-centric trial designs, and the focus on real-world evidence. These trends are transforming how research is conducted and improving overall trial efficiency.
Global CRO Services market
| Segmentation Details | Description |
|---|---|
| Service Type | Clinical Trials, Data Management, Regulatory Affairs, Pharmacovigilance |
| End User | Pharmaceutical Companies, Biotechnology Firms, Medical Device Manufacturers, Academic Institutions |
| Delivery Model | On-site, Remote, Hybrid, Outsourced |
| Therapy Area | Oncology, Neurology, Cardiovascular, Infectious Diseases |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Global CRO Services Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at