444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The Genital Herpes Research and Development Pipeline Market is a critical segment within the pharmaceutical and biotechnology industry, focusing on the discovery, development, and commercialization of novel therapies for the treatment and prevention of genital herpes infections. Genital herpes, caused by the herpes simplex virus (HSV), is a common sexually transmitted infection (STI) with significant global burden, driving the need for innovative therapeutic interventions. This market encompasses a diverse pipeline of investigational drugs, vaccines, and biologics targeting various stages of the herpes virus life cycle, aiming to improve patient outcomes and reduce transmission rates.
Meaning:
The Genital Herpes Research and Development Pipeline Market comprise the ongoing efforts of pharmaceutical companies, research institutions, and academia to advance the understanding of genital herpes pathogenesis, identify potential drug targets, and develop new therapeutic modalities. This market focuses on preclinical and clinical-stage candidates, including small molecule inhibitors, antiviral agents, immunomodulators, and gene editing technologies, aimed at disrupting viral replication, modulating immune responses, and reducing the frequency and severity of genital herpes outbreaks.
Executive Summary:
The Genital Herpes Research and Development Pipeline Market represent a dynamic and rapidly evolving landscape, driven by advancements in virology, immunology, and molecular biology, as well as increasing awareness of the clinical and public health significance of genital herpes infections. This article provides a comprehensive analysis of key market trends, drivers, challenges, and opportunities shaping the Genital Herpes R&D Pipeline Market. From novel antiviral mechanisms to innovative vaccine platforms, the market offers promising prospects for stakeholders seeking to address unmet medical needs in the management and prevention of genital herpes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Genital Herpes Research and Development Pipeline Market operates in a dynamic and competitive environment influenced by factors such as scientific advances, regulatory policies, market competition, and industry partnerships. Market dynamics shape investment priorities, research strategies, product development pipelines, and commercialization strategies for pharmaceutical companies, biotechnology firms, and academic research institutions.
Regional Analysis:
Regional variations exist within the Genital Herpes R&D Pipeline Market due to differences in research funding, academic expertise, regulatory frameworks, and disease epidemiology across geographical regions. Developed regions such as North America and Europe have strong research infrastructure and collaborative networks, while emerging markets in Asia Pacific and Latin America offer growth opportunities for clinical research and development partnerships.
Competitive Landscape:
Leading Companies in Genital Herpes Research and Development Pipeline Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation:
The Genital Herpes R&D Pipeline Market can be segmented based on various factors, including:
Segmentation provides insights into research priorities, clinical development strategies, and market potential for investigational therapies targeting specific stages of genital herpes infection and patient populations.
Category-wise Insights:
Each category within the Genital Herpes R&D Pipeline Market offers unique insights into research priorities, therapeutic approaches, and market trends:
Key Benefits for Industry Participants and Stakeholders:
The Genital Herpes Research and Development Pipeline Market offers several benefits for industry participants and stakeholders, including:
SWOT Analysis:
A SWOT analysis of the Genital Herpes Research and Development Pipeline Market provides insights into its strengths, weaknesses, opportunities, and threats:
Market Key Trends:
Key trends shaping the Genital Herpes R&D Pipeline Market include:
Covid-19 Impact:
The Covid-19 pandemic has impacted the Genital Herpes R&D Pipeline Market in various ways, including:
Key Industry Developments:
Key developments in the Genital Herpes R&D Pipeline Market include:
Analyst Suggestions:
Industry analysts suggest that stakeholders in the Genital Herpes R&D Pipeline Market focus on:
Future Outlook:
The future outlook for the Genital Herpes Research and Development Pipeline Market is optimistic, driven by factors such as increasing research investment, scientific innovation, and clinical trial activity in the field of virology and immunology. As the market continues to evolve, opportunities for therapeutic innovation, personalized medicine, and collaborative research partnerships will emerge, positioning stakeholders to address unmet medical needs and improve patient outcomes in the management and prevention of genital herpes infections.
Conclusion:
In conclusion, the Genital Herpes Research and Development Pipeline Market represent a dynamic and promising area of therapeutic innovation and scientific discovery, driven by the urgent need for effective treatments and preventive interventions for genital herpes infections. From small molecule antivirals to novel vaccine platforms and gene editing technologies, the market offers a diverse pipeline of investigational therapies with the potential to transform patient care and public health outcomes. By leveraging scientific advances, regulatory support, and collaborative partnerships, stakeholders in the Genital Herpes R&D Pipeline Market can advance the development of safe, efficacious, and accessible therapies for genital herpes patients worldwide.
What is Genital Herpes Research and Development Pipeline?
Genital Herpes Research and Development Pipeline refers to the ongoing studies and innovations aimed at developing new treatments and vaccines for genital herpes, a common sexually transmitted infection caused by the herpes simplex virus.
What are the key players in the Genital Herpes Research and Development Pipeline Market?
Key players in the Genital Herpes Research and Development Pipeline Market include companies like GSK, Amgen, and Moderna, which are involved in developing novel therapies and vaccines for genital herpes, among others.
What are the growth factors driving the Genital Herpes Research and Development Pipeline Market?
The growth of the Genital Herpes Research and Development Pipeline Market is driven by increasing prevalence of genital herpes, rising awareness about sexually transmitted infections, and advancements in biotechnology that facilitate innovative treatment options.
What challenges does the Genital Herpes Research and Development Pipeline Market face?
Challenges in the Genital Herpes Research and Development Pipeline Market include regulatory hurdles, the complexity of developing effective vaccines, and the stigma associated with sexually transmitted infections that may hinder research participation.
What opportunities exist in the Genital Herpes Research and Development Pipeline Market?
Opportunities in the Genital Herpes Research and Development Pipeline Market include the potential for breakthrough therapies, increased funding for research initiatives, and collaborations between pharmaceutical companies and research institutions to accelerate development.
What trends are emerging in the Genital Herpes Research and Development Pipeline Market?
Emerging trends in the Genital Herpes Research and Development Pipeline Market include the use of mRNA technology for vaccine development, personalized medicine approaches, and a focus on combination therapies to enhance treatment efficacy.
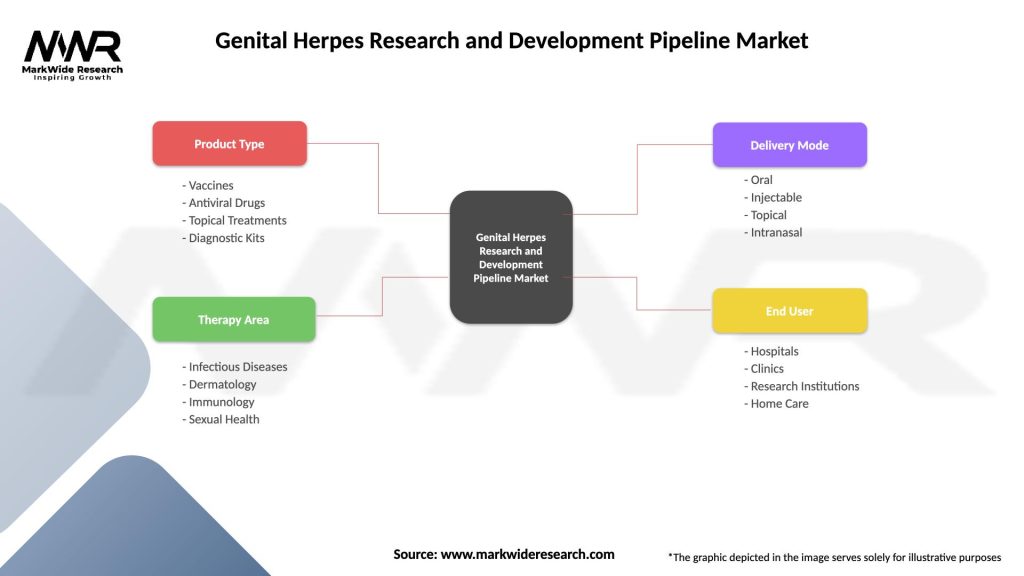
Genital Herpes Research and Development Pipeline Market
| Segmentation Details | Description |
|---|---|
| Product Type | Vaccines, Antiviral Drugs, Topical Treatments, Diagnostic Kits |
| Therapy Area | Infectious Diseases, Dermatology, Immunology, Sexual Health |
| Delivery Mode | Oral, Injectable, Topical, Intranasal |
| End User | Hospitals, Clinics, Research Institutions, Home Care |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Genital Herpes Research and Development Pipeline Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at