444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Gene Vector market refers to the segment of the biotechnology industry that focuses on the development, production, and commercialization of gene delivery systems, also known as vectors. Gene vectors are essential tools in gene therapy, gene editing, and other genetic engineering applications. They are designed to deliver genetic material, such as therapeutic genes or gene editing tools, into target cells to modulate gene expression, correct genetic mutations, or introduce new genetic functions. The Gene Vector market is witnessing significant growth due to the increasing demand for gene therapies and advancements in vector design and delivery technologies.
Meaning
Gene vectors are specialized vehicles used to transport genetic material into cells for various purposes, including therapeutic interventions and genetic engineering applications. They serve as carriers or delivery systems for introducing therapeutic genes, gene editing tools (such as CRISPR-Cas9), or other genetic elements into target cells. Gene vectors can be viral or non-viral in nature, and their selection depends on factors such as target cell type, desired level of gene expression, safety considerations, and specific applications.
Executive Summary
The Gene Vector market is experiencing rapid growth due to the expanding field of gene therapy and genetic engineering. Gene vectors play a pivotal role in the success of these applications by facilitating the delivery of genetic material into target cells. The market is driven by advancements in vector design and delivery technologies, increasing investment in gene therapy research, and a growing number of clinical trials and regulatory approvals. Key players in the market are focused on developing novel vectors with enhanced safety, specificity, and efficiency to address the evolving needs of the gene therapy and genetic engineering sectors.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Dynamics
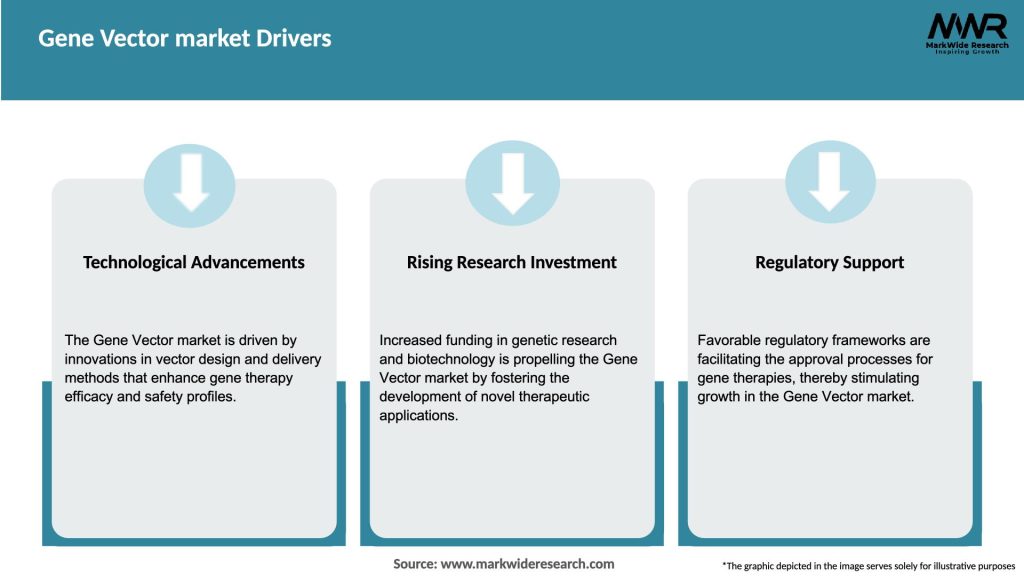
The Gene Vector market is driven by various dynamics, including scientific advancements, regulatory frameworks, patient needs, and industry collaborations. The market is characterized by intense research and development activities, strategic partnerships, and licensing agreements between academic institutions, biotechnology companies, and pharmaceutical manufacturers. Additionally, the evolving regulatory landscape and reimbursement policies for gene therapies significantly impact the market’s growth and commercialization prospects.
Regional Analysis
Competitive Landscape
Leading Companies in the Gene Vector Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
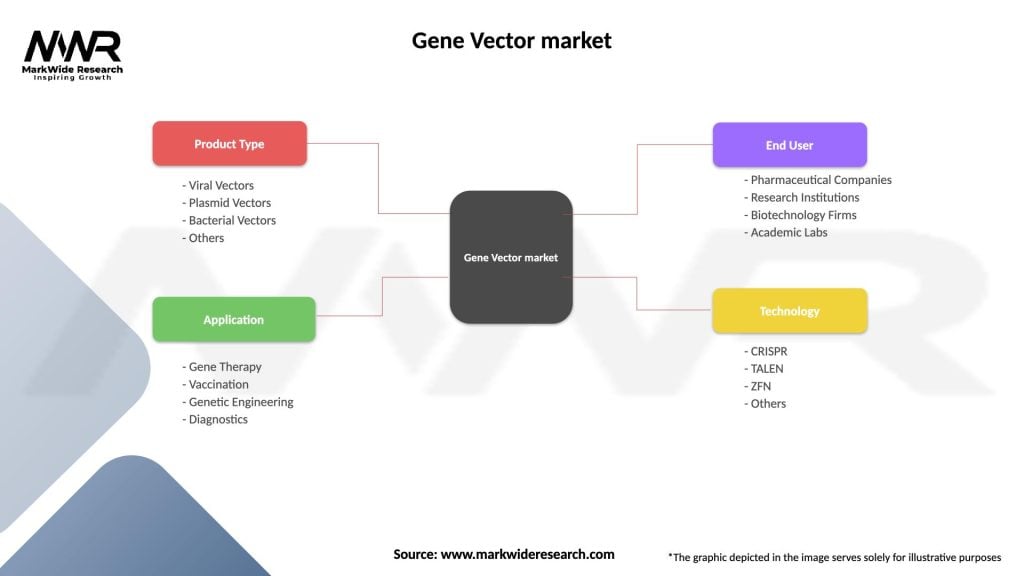
Segmentation
The Gene Vector market can be segmented based on various factors, including vector type, application, and target cell type:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the gene therapy field, including the Gene Vector market. While the pandemic has disrupted research activities and clinical trials, it has also highlighted the importance of innovative medical solutions and accelerated advancements in vector design, manufacturing, and delivery. The pandemic-driven focus on mRNA-based vaccines has further propelled the gene therapy field, as mRNA delivery relies on similar vector technologies.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Gene Vector market is expected to experience substantial growth in the coming years, driven by advancements in vector design, increasing investment in gene therapy research, and a growing pipeline of gene therapies in development. The market will likely witness the development of next-generation vectors with improved safety profiles, enhanced delivery efficiency, and targeted transduction capabilities. Continued collaboration among industry stakeholders, regulatory bodies, and academic institutions will be pivotal in realizing the full potential of gene vectors in gene therapy and genetic engineering.
Conclusion
The Gene Vector market plays a critical role in the advancement of gene therapy and genetic engineering applications. Gene vectors serve as essential tools for delivering therapeutic genes or gene editing tools into target cells. The market is driven by advancements in vector design, increasing investment in gene therapy research, and growing clinical trial activities. However, challenges related to safety, regulatory compliance, and high costs persist. The future of the Gene Vector market looks promising, with opportunities for novel vector development, integration of gene editing technologies, and expanded therapeutic applications. Collaboration, research investment, and regulatory support are key to driving innovation and bringing transformative gene therapies to patients in need.
What is Gene Vector?
Gene vectors are vehicles used to deliver genetic material into cells. They are essential in gene therapy, vaccine development, and genetic research, facilitating the introduction of new genes to treat diseases or enhance biological functions.
Who are the key players in the Gene Vector market?
Key players in the Gene Vector market include companies like Novartis, Gilead Sciences, and Spark Therapeutics, which are involved in developing innovative gene therapies and vector technologies, among others.
What are the main drivers of growth in the Gene Vector market?
The growth of the Gene Vector market is driven by increasing investments in gene therapy research, rising prevalence of genetic disorders, and advancements in vector technology that enhance delivery efficiency.
What challenges does the Gene Vector market face?
The Gene Vector market faces challenges such as regulatory hurdles, potential safety concerns related to vector use, and the complexity of manufacturing high-quality vectors at scale.
What opportunities exist in the Gene Vector market?
Opportunities in the Gene Vector market include the development of novel vectors for targeted therapies, expansion into rare disease treatments, and collaborations between biotech firms and research institutions to innovate vector technologies.
What trends are shaping the Gene Vector market?
Trends in the Gene Vector market include the increasing use of viral vectors for gene delivery, advancements in CRISPR technology, and a growing focus on personalized medicine approaches that utilize gene vectors for tailored therapies.
Gene Vector market
| Segmentation Details | Description |
|---|---|
| Product Type | Viral Vectors, Plasmid Vectors, Bacterial Vectors, Others |
| Application | Gene Therapy, Vaccination, Genetic Engineering, Diagnostics |
| End User | Pharmaceutical Companies, Research Institutions, Biotechnology Firms, Academic Labs |
| Technology | CRISPR, TALEN, ZFN, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Gene Vector Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at