444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview: Gene therapy drugs are designed to introduce, modify, or regulate genes within a patient’s cells to correct genetic abnormalities, restore normal cellular function, or induce therapeutic effects. These drugs encompass a diverse range of modalities, including viral vectors, non-viral vectors, RNA-based therapies, and genome editing techniques such as CRISPR-Cas9. By harnessing the power of molecular biology and genetic engineering, gene therapy drugs offer the potential for targeted and personalized treatments that address the underlying causes of genetic diseases.
Meaning: Gene therapy drugs involve the delivery of therapeutic genes or genetic material into a patient’s cells to correct or modulate dysfunctional genes, restore normal cellular function, or induce therapeutic effects. These drugs can be administered through various routes, including intravenous injection, direct tissue injection, or ex vivo manipulation of patient-derived cells. Gene therapy represents a paradigm shift in medicine, offering the potential to cure or manage genetic diseases that were previously considered incurable or untreatable.
Executive Summary: The gene therapy drugs market is experiencing rapid growth driven by scientific advancements, clinical successes, and increasing commercialization efforts by pharmaceutical companies. Key market players are investing heavily in research and development to expand the therapeutic applications of gene therapy, optimize delivery systems, and overcome technical challenges such as immune responses and off-target effects. With a growing pipeline of gene therapy candidates and regulatory approvals, the market is poised for significant expansion in the coming years.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The gene therapy drugs market is characterized by dynamic interactions between scientific progress, regulatory frameworks, and market forces. Market players need to navigate these dynamics by leveraging interdisciplinary expertise, strategic partnerships, and adaptive business models to capitalize on emerging opportunities and address evolving challenges in the global market.
Regional Analysis: The gene therapy drugs market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory environments, and market access pathways. North America leads the market due to a strong biotechnology sector, supportive regulatory agencies such as the FDA, and a favorable reimbursement landscape for innovative therapies. Europe and Asia Pacific are also significant markets, with increasing investments in gene therapy research, clinical infrastructure, and market expansion initiatives.
Competitive Landscape:
Leading Companies in Gene Therapy Drugs Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
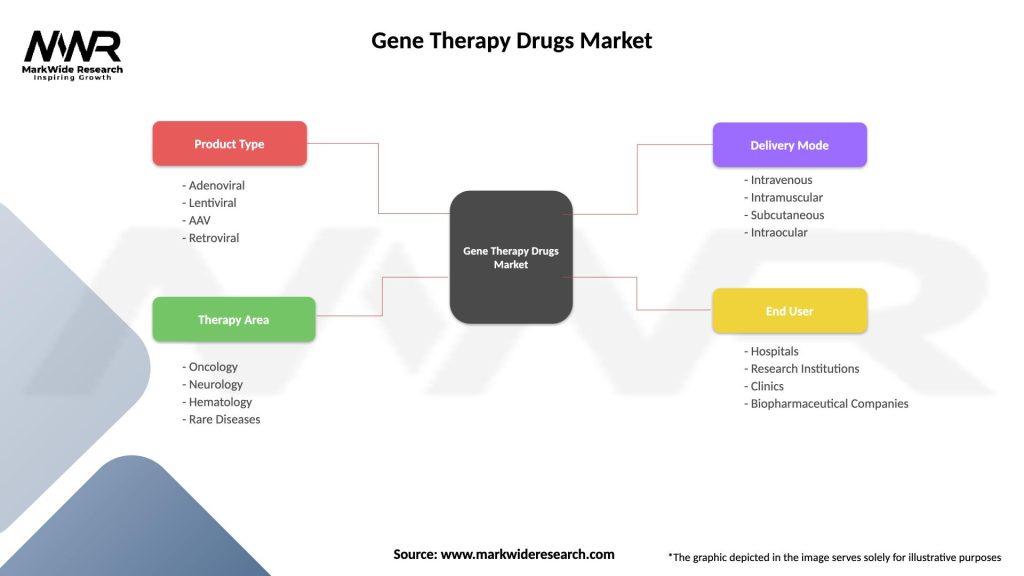
Segmentation: The gene therapy drugs market can be segmented based on therapeutic indication, vector type, delivery method, and geography. Therapeutic indications include genetic disorders, cancer, cardiovascular diseases, neurological disorders, and infectious diseases. Vector types encompass viral vectors (e.g., adenovirus, lentivirus, adeno-associated virus) and non-viral vectors (e.g., naked DNA, RNA nanoparticles). Delivery methods range from systemic administration to localized injection, ex vivo cell therapy, and in situ gene editing approaches.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has accelerated the development and adoption of gene therapy approaches for infectious diseases, vaccine development, and immunomodulation strategies. The crisis has underscored the importance of innovative medical technologies, collaborative research networks, and resilient healthcare systems in addressing global health emergencies and pandemics.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The gene therapy drugs market is poised for continued growth driven by scientific innovation, regulatory support, and increasing market acceptance of gene-based therapies. With a growing pipeline of candidates, expanding therapeutic applications, and improving manufacturing technologies, gene therapy is expected to play an increasingly prominent role in the future of healthcare, offering transformative solutions for genetic diseases, cancer, infectious diseases, and chronic conditions.
Conclusion: Gene therapy drugs represent a paradigm shift in medicine, offering the potential to cure or manage genetic diseases and disorders at the molecular level. By harnessing the power of genetic engineering, molecular biology, and personalized medicine, gene therapy holds the promise of transforming the treatment landscape for a wide range of conditions, from rare genetic disorders to common chronic diseases. With ongoing advancements in technology, research, and clinical practice, gene therapy is poised to revolutionize healthcare and improve patient outcomes in the 21st century.
What is Gene Therapy Drugs?
Gene therapy drugs are medical treatments that involve altering the genes inside a patient’s cells to treat or prevent disease. This innovative approach can be used to address genetic disorders, certain types of cancer, and viral infections by correcting defective genes or introducing new ones.
What are the key players in the Gene Therapy Drugs Market?
Key players in the Gene Therapy Drugs Market include Novartis, Gilead Sciences, and Spark Therapeutics, among others. These companies are at the forefront of developing and commercializing gene therapies for various medical conditions.
What are the main drivers of growth in the Gene Therapy Drugs Market?
The growth of the Gene Therapy Drugs Market is driven by advancements in genetic research, increasing prevalence of genetic disorders, and rising investments in biotechnology. Additionally, the potential for personalized medicine is fueling interest and development in this field.
What challenges does the Gene Therapy Drugs Market face?
The Gene Therapy Drugs Market faces challenges such as high development costs, regulatory hurdles, and ethical concerns regarding gene editing. These factors can hinder the pace of innovation and market entry for new therapies.
What opportunities exist in the Gene Therapy Drugs Market?
Opportunities in the Gene Therapy Drugs Market include the potential for new treatment modalities for rare diseases, collaborations between biotech firms and academic institutions, and the expansion of gene therapies into broader therapeutic areas. This could lead to significant advancements in patient care.
What trends are shaping the Gene Therapy Drugs Market?
Trends in the Gene Therapy Drugs Market include the rise of CRISPR technology for gene editing, increased focus on patient-centric approaches, and the development of combination therapies. These trends are expected to enhance the effectiveness and accessibility of gene therapies.
Gene Therapy Drugs Market
| Segmentation Details | Description |
|---|---|
| Product Type | Adenoviral, Lentiviral, AAV, Retroviral |
| Therapy Area | Oncology, Neurology, Hematology, Rare Diseases |
| Delivery Mode | Intravenous, Intramuscular, Subcutaneous, Intraocular |
| End User | Hospitals, Research Institutions, Clinics, Biopharmaceutical Companies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Gene Therapy Drugs Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at