444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The Ganciclovir API Market revolves around the production and distribution of Active Pharmaceutical Ingredients (APIs) used in the formulation of Ganciclovir-based medications. Ganciclovir is an antiviral medication primarily used for the treatment of cytomegalovirus (CMV) infections, particularly in immunocompromised patients such as those with HIV/AIDS or undergoing organ transplantation. The API market plays a crucial role in ensuring the availability and quality of Ganciclovir for pharmaceutical manufacturers worldwide.
Meaning:
Ganciclovir API refers to the active pharmaceutical ingredient used in the formulation of Ganciclovir-based medications. It is a synthetic nucleoside analog that inhibits the replication of CMV by interfering with viral DNA synthesis. Ganciclovir API is produced through complex chemical processes and must meet stringent quality standards to ensure the safety and efficacy of the final drug products.
Executive Summary:
The Ganciclovir API Market is a vital segment of the pharmaceutical industry, driven by the increasing prevalence of CMV infections and the growing demand for antiviral medications. Pharmaceutical companies rely on reliable sources of high-quality Ganciclovir API to manufacture formulations for the treatment and prevention of CMV-related diseases. The market is characterized by stringent regulatory requirements, technological advancements, and strategic collaborations among key players.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Ganciclovir API Market operates within a dynamic landscape shaped by factors such as disease epidemiology, regulatory frameworks, technological advancements, and market competition. API manufacturers, pharmaceutical companies, healthcare providers, and regulatory agencies navigate these dynamics to ensure the availability, affordability, and quality of Ganciclovir-based medications for patients worldwide.
Regional Analysis:
The demand for Ganciclovir API varies across different geographic regions, influenced by factors such as disease prevalence, healthcare infrastructure, regulatory requirements, and market competition. Regions with high incidences of CMV infections, such as North America, Europe, and parts of Asia-Pacific, demonstrate robust demand for Ganciclovir API, while emerging markets in Latin America, Africa, and the Middle East present growth opportunities for market expansion.
Competitive Landscape:
Leading Companies: Ganciclovir API Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
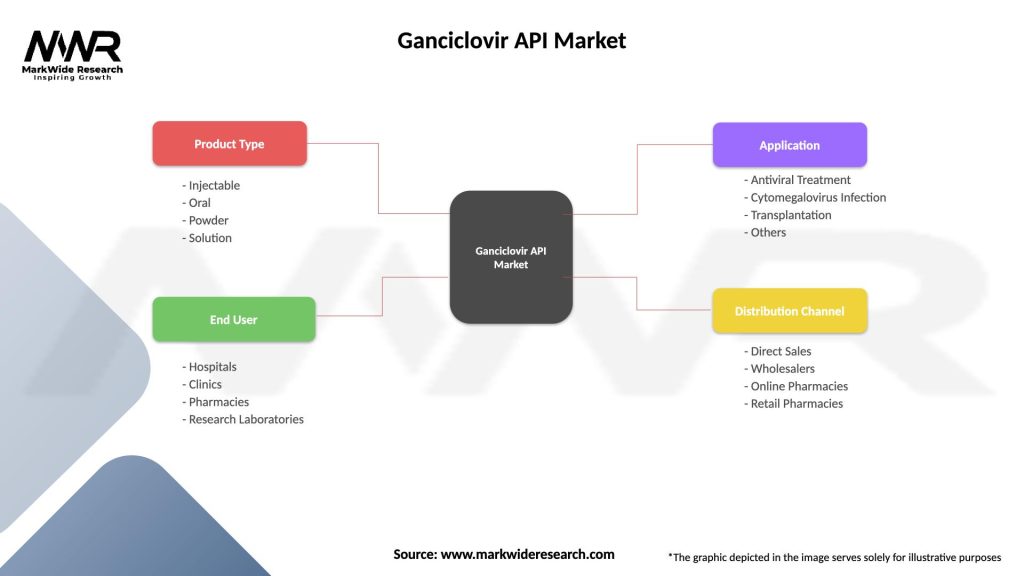
Segmentation:
The Ganciclovir API Market can be segmented based on:
Segmentation provides insights into market dynamics, customer preferences, and competitive strategies, enabling companies to tailor their products and services to specific market segments and geographic regions.
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has influenced the Ganciclovir API Market by:
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The future outlook for the Ganciclovir API Market is optimistic, with opportunities for growth and innovation driven by:
Conclusion:
In conclusion, the Ganciclovir API Market plays a critical role in the pharmaceutical industry, providing essential ingredients for the formulation of Ganciclovir-based medications used in the treatment of CMV infections. Despite challenges such as regulatory hurdles, patent protections, and supply chain disruptions, the market demonstrates resilience and opportunities for growth driven by research advancements, regulatory flexibility, and market expansion. By embracing innovation, collaboration, and regulatory compliance, stakeholders can navigate the evolving landscape, address unmet medical needs, and contribute to the advancement of antiviral therapies and patient care.
What is Ganciclovir API?
Ganciclovir API refers to the active pharmaceutical ingredient used in the production of Ganciclovir, an antiviral medication primarily used to treat cytomegalovirus infections in immunocompromised patients. It is crucial in the management of viral infections, particularly in organ transplant recipients and individuals with HIV/AIDS.
What are the key players in the Ganciclovir API Market?
Key players in the Ganciclovir API Market include companies such as Hetero Labs, Cipla, and Teva Pharmaceutical Industries, which are known for their production and supply of antiviral medications. These companies contribute significantly to the availability and distribution of Ganciclovir API globally, among others.
What are the growth factors driving the Ganciclovir API Market?
The Ganciclovir API Market is driven by the increasing prevalence of cytomegalovirus infections, particularly among immunocompromised populations. Additionally, the rising demand for effective antiviral therapies and advancements in pharmaceutical manufacturing processes are contributing to market growth.
What challenges does the Ganciclovir API Market face?
The Ganciclovir API Market faces challenges such as stringent regulatory requirements and the high cost of production. Additionally, competition from generic alternatives and potential supply chain disruptions can impact market stability.
What opportunities exist in the Ganciclovir API Market?
Opportunities in the Ganciclovir API Market include the potential for research and development of new formulations and combination therapies. Furthermore, expanding healthcare access in developing regions presents a significant opportunity for market growth.
What trends are shaping the Ganciclovir API Market?
Trends in the Ganciclovir API Market include the increasing focus on personalized medicine and the development of novel drug delivery systems. Additionally, the rise of biotechnology and biopharmaceuticals is influencing the production and application of Ganciclovir API.
Ganciclovir API Market
| Segmentation Details | Description |
|---|---|
| Product Type | Injectable, Oral, Powder, Solution |
| End User | Hospitals, Clinics, Pharmacies, Research Laboratories |
| Application | Antiviral Treatment, Cytomegalovirus Infection, Transplantation, Others |
| Distribution Channel | Direct Sales, Wholesalers, Online Pharmacies, Retail Pharmacies |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies: Ganciclovir API Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at