444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The Favipiravir market is experiencing significant growth due to its effectiveness in treating viral infections, particularly in the context of the COVID-19 pandemic. Favipiravir, also known as Avigan, is an antiviral medication that inhibits the replication of RNA viruses. It has shown promising results in clinical trials, leading to its widespread adoption in the treatment of COVID-19 patients. This market analysis provides insights into the current state of the Favipiravir market, its key drivers, restraints, opportunities, and future outlook.
Favipiravir is a pharmaceutical compound developed by Toyama Chemical (a subsidiary of Fujifilm) that exhibits antiviral properties. It was initially developed to treat influenza and other RNA viral infections. The mechanism of action involves inhibiting the RNA-dependent RNA polymerase enzyme, essential for viral replication. Due to its broad-spectrum antiviral activity, Favipiravir has gained attention for its potential in combating emerging viral outbreaks, including the ongoing COVID-19 pandemic.
Executive Summary:
The Favipiravir market has witnessed significant growth in recent years, primarily driven by the global demand for effective COVID-19 treatments. The market is expected to continue expanding as the prevalence of viral infections persists. Key market insights highlight the growing adoption of Favipiravir, the emergence of generic versions, increasing research and development activities, and strategic collaborations between pharmaceutical companies to meet the demand.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Favipiravir market is driven by the interplay of various factors. The increasing prevalence of viral infections, particularly during the COVID-19 pandemic, has accelerated the demand for effective antiviral treatments. Regulatory approvals and emergency use authorizations have facilitated the availability of Favipiravir in many regions. However, limited awareness, stringent regulations, and potential side effects act as barriers to market growth. Opportunities lie in expanding usage, exploring new indications, and partnerships for efficient distribution.
Regional Analysis:
The Favipiravir market exhibits regional variations in terms of adoption, regulatory landscape, and market potential. North America and Europe currently dominate the market due to early regulatory approvals and higher healthcare expenditure. The Asia-Pacific region, especially countries like India, Japan, and China, present significant opportunities due to their large population, high disease burden, and growing healthcare infrastructure. Emerging economies in Latin America and Africa are also expected to contribute to market growth.
Competitive Landscape:
Leading Companies in the Favipiravir Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

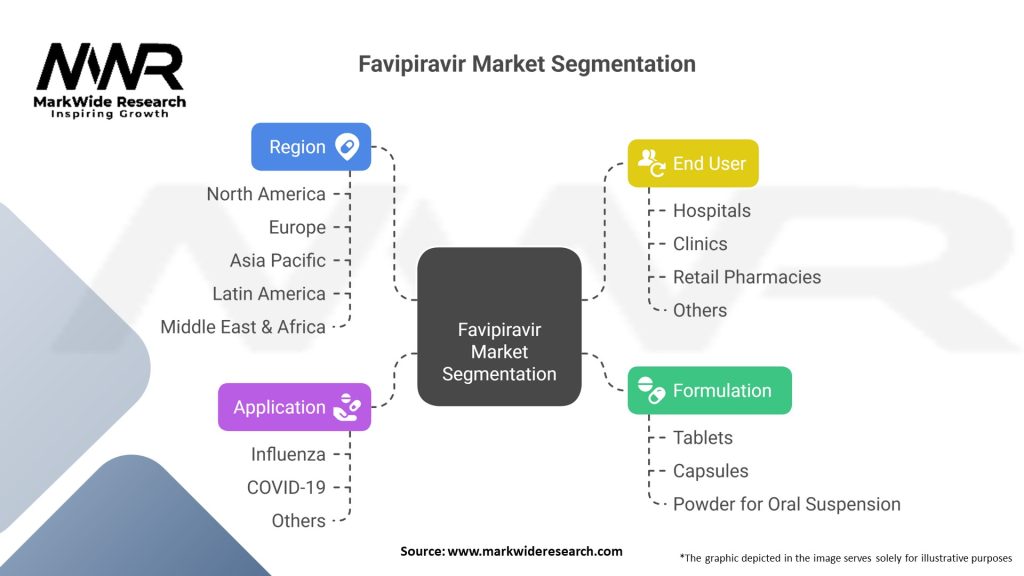
Segmentation:
The Favipiravir market can be segmented based on end-user and distribution channel. In terms of end-users, the market caters to hospitals, clinics, and retail pharmacies. The distribution channel segment includes online pharmacies, hospital pharmacies, and retail pharmacies. Different end-users and distribution channels require specific marketing and distribution strategies to ensure efficient access to Favipiravir.
Category-wise Insights:
Favipiravir falls under the category of antiviral medications. It is specifically known for its efficacy against RNA viruses, including influenza and the SARS-CoV-2 virus responsible for COVID-19. The drug has gained significant attention as a potential treatment option during viral outbreaks due to its mechanism of action and broad-spectrum antiviral activity.
Key Benefits for Industry Participants and Stakeholders:
Industry participants and stakeholders in the Favipiravir market can reap several benefits. These include:
SWOT Analysis:
Market Key Trends:
Several key trends shape the Favipiravir market:
Covid-19 Impact:
The COVID-19 pandemic has had a significant impact on the Favipiravir market. The urgent need for effective treatments against the SARS-CoV-2 virus has led to the rapid adoption of Favipiravir in many countries. Regulatory authorities have granted emergency use authorizations, expediting its availability. The increased demand for Favipiravir, both as a standalone treatment and in combination therapies, has contributed to the growth of the market. The ongoing pandemic has highlighted the importance of antiviral medications like Favipiravir in managing viral outbreaks and has created opportunities for further research and development in the field.
Key Industry Developments:
The Favipiravir market has witnessed several key industry developments, including:
Analyst Suggestions:
Based on the market analysis, analysts suggest the following:
Future Outlook:
The future outlook for the Favipiravir market remains positive. The continued prevalence of viral infections, including outbreaks of novel viruses, drives the demand for effective antiviral medications like Favipiravir. Ongoing research and clinical trials will contribute to a deeper understanding of the drug’s potential and expand its application beyond COVID-19. Additionally, partnerships, collaborations, and strategic investments in manufacturing capacities will support market growth.
Conclusion:
The Favipiravir market has experienced significant growth due to its effectiveness in treating viral infections, particularly COVID-19. With its broad-spectrum antiviral activity and promising results in clinical trials, Favipiravir has gained attention as a potential treatment option. However, limited awareness, stringent regulations, and potential side effects pose challenges to its widespread adoption. Industry participants should focus on increasing awareness, expanding market reach, and exploring collaborations to harness the full potential of Favipiravir in combating viral outbreaks and improving global public health.
What is Favipiravir?
Favipiravir is an antiviral medication primarily used to treat influenza and has been investigated for its efficacy against other viral infections, including COVID-19. It works by inhibiting viral RNA polymerase, thereby preventing the replication of the virus.
Who are the key players in the Favipiravir Market?
Key players in the Favipiravir Market include Fujifilm Toyama Chemical, Glenmark Pharmaceuticals, and Hetero Labs, among others. These companies are involved in the production and distribution of Favipiravir for various therapeutic applications.
What are the growth factors driving the Favipiravir Market?
The growth of the Favipiravir Market is driven by the increasing prevalence of viral infections, the ongoing need for effective antiviral treatments, and the rising research and development activities in the pharmaceutical sector. Additionally, the global health crisis has accelerated the demand for antiviral medications.
What challenges does the Favipiravir Market face?
The Favipiravir Market faces challenges such as regulatory hurdles, potential side effects associated with the drug, and competition from other antiviral therapies. These factors can impact market growth and acceptance among healthcare providers.
What opportunities exist in the Favipiravir Market?
Opportunities in the Favipiravir Market include expanding its applications beyond influenza and COVID-19, such as in the treatment of other viral infections. Additionally, increasing collaborations between pharmaceutical companies and research institutions can enhance innovation and market reach.
What trends are shaping the Favipiravir Market?
Trends shaping the Favipiravir Market include the growing focus on antiviral drug development, advancements in drug formulation technologies, and the increasing use of telemedicine for patient management. These trends are likely to influence how Favipiravir is utilized in clinical settings.
Favipiravir Market:
| Segmentation | Details |
|---|---|
| Formulation | Tablets, Capsules, Powder for Oral Suspension |
| Application | Influenza, COVID-19, Others |
| End User | Hospitals, Clinics, Retail Pharmacies, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Favipiravir Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at