444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Ewing sarcoma therapeutics pipeline market refers to the ongoing research and development activities aimed at discovering and developing innovative therapies for the treatment of Ewing sarcoma, a rare form of bone cancer that primarily affects children and young adults. This market overview provides a comprehensive analysis of the Ewing sarcoma therapeutics pipeline market, including its meaning, executive summary, key market insights, market drivers, market restraints, market opportunities, market dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, key benefits for industry participants and stakeholders, SWOT analysis, market key trends, Covid-19 impact, key industry developments, analyst suggestions, future outlook, and conclusion.
Meaning
Ewing sarcoma is a rare type of cancer that arises in the bones or soft tissues, typically affecting children and young adults. The Ewing sarcoma therapeutics pipeline refers to the collection of drugs and therapies that are currently undergoing various stages of research, preclinical testing, and clinical trials for potential use in the treatment of Ewing sarcoma. The pipeline represents the future potential of novel treatment options that may improve patient outcomes and survival rates.
Executive Summary
The Ewing sarcoma therapeutics pipeline market is witnessing significant research and development efforts focused on discovering and developing new treatment options for Ewing sarcoma. The pipeline consists of various drugs and therapies, including targeted therapies, immunotherapies, and combination therapies, that aim to address the unmet medical needs of Ewing sarcoma patients. Although challenges such as limited treatment options, high development costs, and stringent regulatory requirements exist, the pipeline offers hope for improved outcomes and increased survival rates for patients with this rare disease.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
The Ewing sarcoma therapeutics pipeline market is driven by several factors:
Market Restraints
Despite the positive growth factors, the Ewing sarcoma therapeutics pipeline market faces some challenges:
Market Opportunities
The Ewing sarcoma therapeutics pipeline presents several opportunities for growth and advancement:
Market Dynamics
The Ewing sarcoma therapeutics pipeline market is influenced by various dynamic factors, including scientific advancements, regulatory landscape, research funding, and patient advocacy. Market dynamics also encompass the collaboration between stakeholders, the integration of new technologies, and the evolving understanding of the underlying biology and genetic drivers of Ewing sarcoma.
Regional Analysis
The Ewing sarcoma therapeutics pipeline market can be analyzed on a regional level, considering different geographical regions such as North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. Each region has its own research capabilities, healthcare infrastructure, regulatory frameworks, and patient population characteristics. Regional analysis helps identify market trends, opportunities, and challenges specific to each region.
Competitive Landscape
Leading Companies in the Ewing Sarcoma Therapeutics Pipeline Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
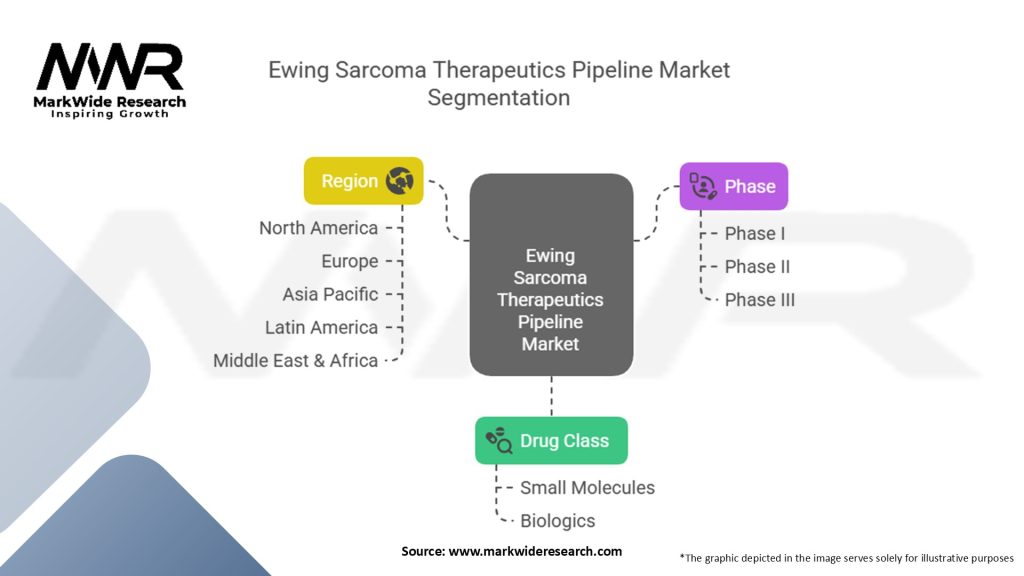
Segmentation
The Ewing sarcoma therapeutics pipeline market can be segmented based on different criteria, including drug class, stage of development, mechanism of action, and target population. By drug class, the pipeline includes targeted therapies, immunotherapies, chemotherapy drugs, and supportive care medications. By stage of development, the pipeline can be categorized as preclinical, Phase I, Phase II, and Phase III. By mechanism of action, the pipeline comprises drugs that inhibit specific molecular targets, modulate the immune system, or disrupt cancer cell processes. By target population, the pipeline addresses different age groups, molecular subtypes, and disease stages.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The Ewing sarcoma therapeutics pipeline offers several benefits for industry participants and stakeholders:
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had an impact on the Ewing sarcoma therapeutics pipeline, causing disruptions in clinical trials, research funding, and patient care. However, the pandemic has also highlighted the need for innovative therapies and accelerated the adoption of technologies such as virtual clinical trials and telemedicine, which may have long-term benefits for drug development and patient access to therapies.
Key Industry Developments
Analyst Suggestions
Based on market analysis and trends, analysts suggest the following strategies for industry participants:
Future Outlook
The future outlook for the Ewing sarcoma therapeutics pipeline market is promising, with ongoing advancements in research, technology, and treatment approaches. The pipeline offers hope for improved therapeutic options, personalized treatment strategies, and increased survival rates for Ewing sarcoma patients. The integration of targeted therapies, immunotherapies, and combination approaches holds significant potential in transforming the treatment landscape. Continued investment in research, collaborations, and patient-centric approaches will drive the progress of the pipeline and contribute to improved patient outcomes.
Conclusion
The Ewing sarcoma therapeutics pipeline market represents a beacon of hope for patients with this rare form of bone cancer. Ongoing research and development efforts are focused on discovering and advancing innovative therapies, including targeted therapies and immunotherapies, to address the unmet medical needs of Ewing sarcoma patients.
While challenges such as limited treatment options, high development costs, and regulatory requirements exist, the pipeline offers opportunities for growth, collaboration, and scientific advancements. The future of the Ewing sarcoma therapeutics pipeline market looks promising, with the potential to transform the treatment landscape and improve patient outcomes.
What is Ewing Sarcoma Therapeutics Pipeline?
Ewing Sarcoma Therapeutics Pipeline refers to the development and progression of treatments specifically targeting Ewing Sarcoma, a rare type of cancer that primarily affects children and young adults. This pipeline includes various therapeutic approaches such as chemotherapy, targeted therapy, and immunotherapy.
What are the key companies in the Ewing Sarcoma Therapeutics Pipeline Market?
Key companies involved in the Ewing Sarcoma Therapeutics Pipeline Market include Eli Lilly and Company, Novartis, and Bayer, among others. These companies are engaged in developing innovative therapies and conducting clinical trials to advance treatment options for Ewing Sarcoma.
What are the growth factors driving the Ewing Sarcoma Therapeutics Pipeline Market?
The growth of the Ewing Sarcoma Therapeutics Pipeline Market is driven by increasing incidence rates of Ewing Sarcoma, advancements in biotechnology, and a growing focus on personalized medicine. Additionally, ongoing research and development efforts are enhancing treatment efficacy.
What challenges does the Ewing Sarcoma Therapeutics Pipeline Market face?
The Ewing Sarcoma Therapeutics Pipeline Market faces challenges such as high costs of drug development, regulatory hurdles, and the rarity of the disease which can limit patient recruitment for clinical trials. These factors can slow down the progress of new therapies.
What opportunities exist in the Ewing Sarcoma Therapeutics Pipeline Market?
Opportunities in the Ewing Sarcoma Therapeutics Pipeline Market include the potential for novel therapies that target specific genetic mutations and the expansion of combination therapies. Additionally, increased funding for cancer research presents avenues for innovation.
What trends are emerging in the Ewing Sarcoma Therapeutics Pipeline Market?
Emerging trends in the Ewing Sarcoma Therapeutics Pipeline Market include the rise of immunotherapy and targeted therapies, as well as the use of advanced genomic technologies to identify biomarkers. These trends are shaping the future of treatment strategies for Ewing Sarcoma.
Ewing Sarcoma Therapeutics Pipeline Market
| Segmentation | Details |
|---|---|
| Drug Class | Small Molecules, Biologics |
| Phase | Phase I, Phase II, Phase III |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Ewing Sarcoma Therapeutics Pipeline Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at