444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview:
The Europe Vascular Access Device Market is witnessing substantial growth, driven by the increasing prevalence of chronic diseases, rising demand for minimally invasive procedures, and advancements in medical technology. Vascular access devices (VADs) are medical devices used to access and manage the vascular system for various medical treatments, such as intravenous therapy, blood sampling, and drug administration. With the aging population and the growing need for long-term medical treatments, the demand for vascular access devices has surged across hospitals, clinics, and home healthcare settings in Europe. The market is experiencing continuous innovation in catheter design, materials, and infection control measures to improve patient outcomes and ensure patient safety.
Meaning:
Vascular Access Devices (VADs) refer to medical devices used to access the bloodstream and provide a conduit for administering medications, fluids, or blood products. These devices are crucial for patients who require long-term medical treatments, such as chemotherapy, dialysis, or parenteral nutrition. VADs include central venous catheters, peripherally inserted central catheters (PICCs), peripheral intravenous catheters, and implantable ports. These devices offer healthcare professionals an efficient and safe means of accessing the vascular system, reducing the need for repeated needle sticks and minimizing patient discomfort during medical procedures.
Executive Summary:
The Europe Vascular Access Device Market is experiencing significant growth, fueled by the rising prevalence of chronic diseases and the increasing adoption of minimally invasive medical procedures. Vascular access devices play a crucial role in managing patient care and improving treatment outcomes in various healthcare settings. The market is characterized by continuous product innovations, the incorporation of infection control measures, and the integration of advanced technologies. Despite challenges related to device-related infections and the need for skilled healthcare professionals, the vascular access device market in Europe is expected to maintain its positive trajectory.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
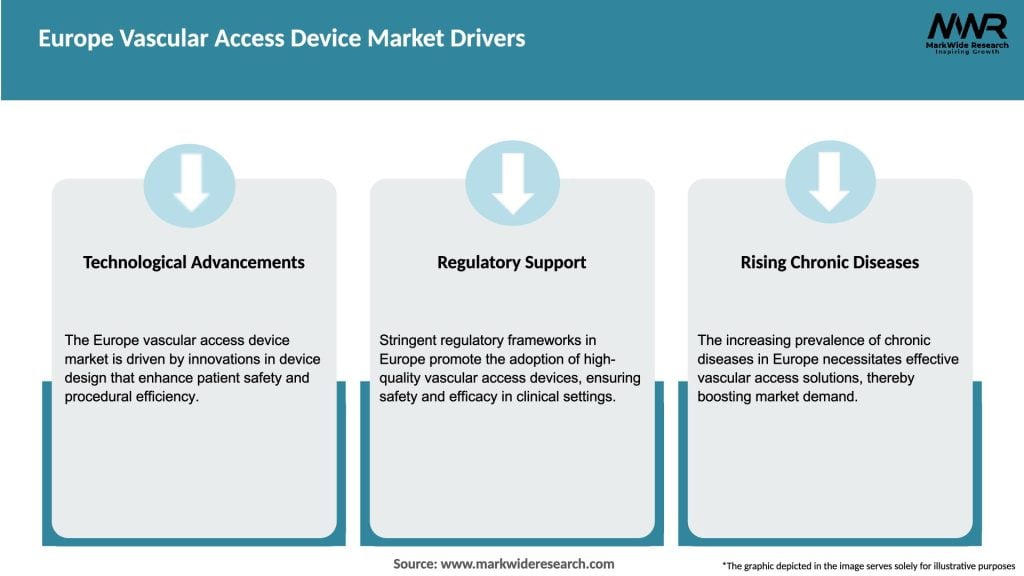
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Europe Vascular Access Device Market is driven by the increasing prevalence of chronic diseases and the growing adoption of minimally invasive medical procedures. Continuous technological advancements and the integration of infection control measures shape the market dynamics.
Regional Analysis:
The Europe Vascular Access Device Market exhibits regional variations due to differences in healthcare infrastructure, disease prevalence, and regulatory frameworks. Key regions such as Germany, the United Kingdom, France, Italy, and Spain are significant contributors to market growth.
Competitive Landscape:
Leading Companies in Europe Vascular Access Device Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
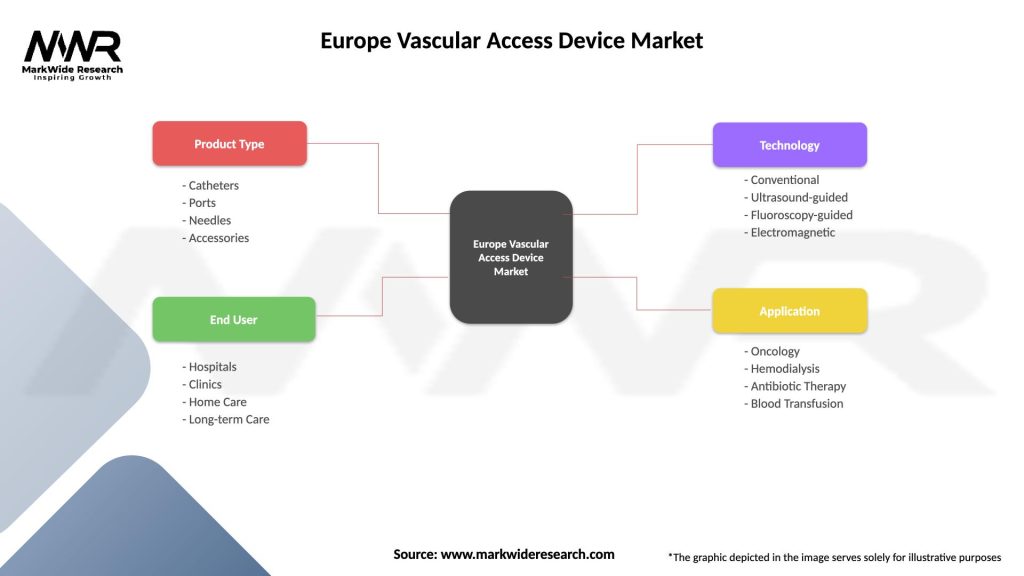
Segmentation:
The Europe Vascular Access Device Market can be segmented based on:
Category-wise Insights:
Central Venous Catheters (CVCs):
Peripheral Intravenous Catheters (PIVCs):
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic had varying effects on the Europe Vascular Access Device Market. While there was an increased demand for VADs in critical care settings, elective procedures were temporarily impacted.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The Europe Vascular Access Device Market is expected to experience continued growth as the prevalence of chronic diseases rises and healthcare providers adopt minimally invasive procedures. Technological advancements and the emphasis on infection control measures will drive market expansion.
Conclusion:
The Europe Vascular Access Device Market is witnessing significant growth, fueled by the increasing prevalence of chronic diseases and the need for minimally invasive medical procedures. Vascular access devices play a vital role in managing patient care and improving treatment outcomes in various healthcare settings. The market’s future looks promising with continuous product innovations, the integration of infection control measures, and the growing emphasis on patient comfort and safety. As Europe’s healthcare sector embraces advanced medical technologies, vascular access devices will continue to play a pivotal role in optimizing patient care and enhancing healthcare delivery.
What is Vascular Access Device?
A Vascular Access Device is a medical instrument used to access the vascular system for various purposes, including administering medications, fluids, or drawing blood. These devices are essential in hospitals and clinics for patients requiring long-term treatment or frequent blood draws.
What are the key players in the Europe Vascular Access Device Market?
Key players in the Europe Vascular Access Device Market include Becton, Dickinson and Company, Medtronic, and Smiths Medical, among others. These companies are known for their innovative products and extensive distribution networks in the healthcare sector.
What are the growth factors driving the Europe Vascular Access Device Market?
The growth of the Europe Vascular Access Device Market is driven by the increasing prevalence of chronic diseases, the rising number of surgical procedures, and advancements in medical technology. Additionally, the demand for minimally invasive procedures is contributing to market expansion.
What challenges does the Europe Vascular Access Device Market face?
The Europe Vascular Access Device Market faces challenges such as stringent regulatory requirements, the risk of infections associated with device use, and the high cost of advanced vascular access technologies. These factors can hinder market growth and adoption.
What opportunities exist in the Europe Vascular Access Device Market?
Opportunities in the Europe Vascular Access Device Market include the development of innovative devices that enhance patient safety and comfort, as well as the expansion of telemedicine and home healthcare services. These trends are likely to create new avenues for growth.
What trends are shaping the Europe Vascular Access Device Market?
Trends shaping the Europe Vascular Access Device Market include the increasing adoption of smart vascular access devices, the integration of digital health technologies, and a focus on patient-centered care. These innovations aim to improve the efficiency and effectiveness of vascular access procedures.
Europe Vascular Access Device Market
| Segmentation Details | Description |
|---|---|
| Product Type | Catheters, Ports, Needles, Accessories |
| End User | Hospitals, Clinics, Home Care, Long-term Care |
| Technology | Conventional, Ultrasound-guided, Fluoroscopy-guided, Electromagnetic |
| Application | Oncology, Hemodialysis, Antibiotic Therapy, Blood Transfusion |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Europe Vascular Access Device Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at