444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Europe Stem Cell Therapy market is experiencing significant growth as advancements in regenerative medicine continue to drive the demand for innovative treatment options. Stem cell therapy involves the use of stem cells to repair, replace, or regenerate damaged tissues and organs, offering potential solutions for various chronic diseases and conditions. As Europe faces an aging population and increasing healthcare needs, stem cell therapy presents a promising avenue for improving patient outcomes and quality of life. The market’s expansion is driven by factors such as rising prevalence of chronic diseases, increasing investments in research and development, and supportive regulatory environment.
Meaning
Stem Cell Therapy refers to the use of stem cells to treat or prevent diseases and injuries by promoting the repair and regeneration of damaged tissues and organs. Stem cells are undifferentiated cells capable of transforming into specialized cells with specific functions. They hold immense potential in regenerative medicine, as they can replace damaged cells and promote tissue healing. Stem cell therapy holds promise for a wide range of medical conditions, including neurodegenerative diseases, cardiovascular disorders, and orthopedic injuries.
Executive Summary
The Europe Stem Cell Therapy market is witnessing robust growth as the medical community embraces regenerative medicine and personalized treatment approaches. This report provides a comprehensive analysis of the market, offering key insights, market drivers, restraints, opportunities, and dynamics influencing its growth. Additionally, it explores regional analysis, competitive landscape, segmentation, category-wise insights, key industry developments, and future outlook. The report concludes with analyst suggestions for industry participants and stakeholders to capitalize on the market’s potential.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
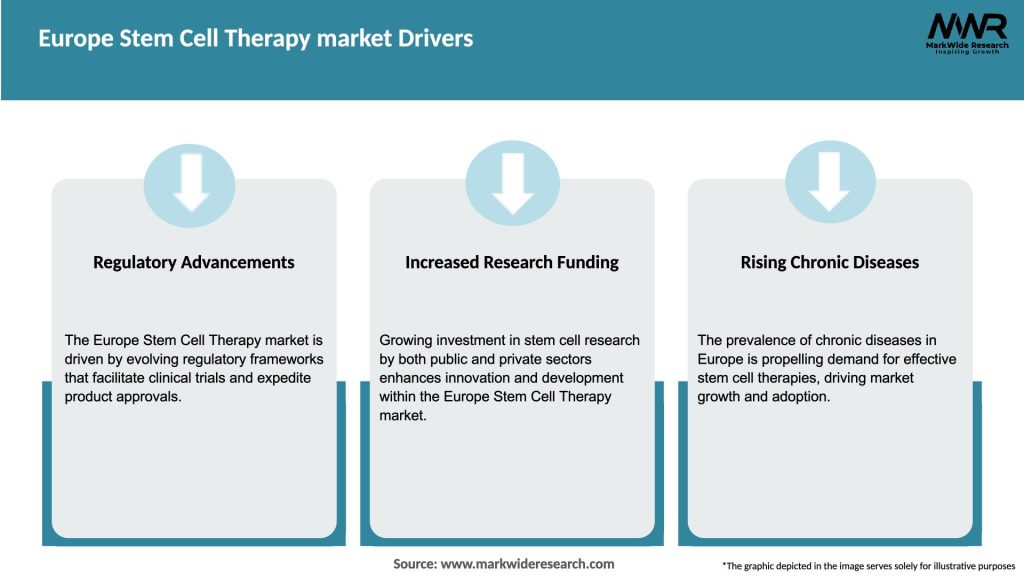
Market Drivers
Several factors are contributing to the growth of the Europe Stem Cell Therapy Market:
Market Restraints
While the Europe Stem Cell Therapy Market shows great promise, there are several challenges that may hinder its growth:
Market Opportunities
The Europe Stem Cell Therapy Market presents numerous opportunities for growth and innovation:
Market Dynamics
The market dynamics of the Europe Stem Cell Therapy Market are influenced by several key factors:
Regional Analysis
The Europe Stem Cell Therapy Market is diverse, with different regions exhibiting unique characteristics:
Competitive Landscape
Leading Companies in Europe Stem Cell Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
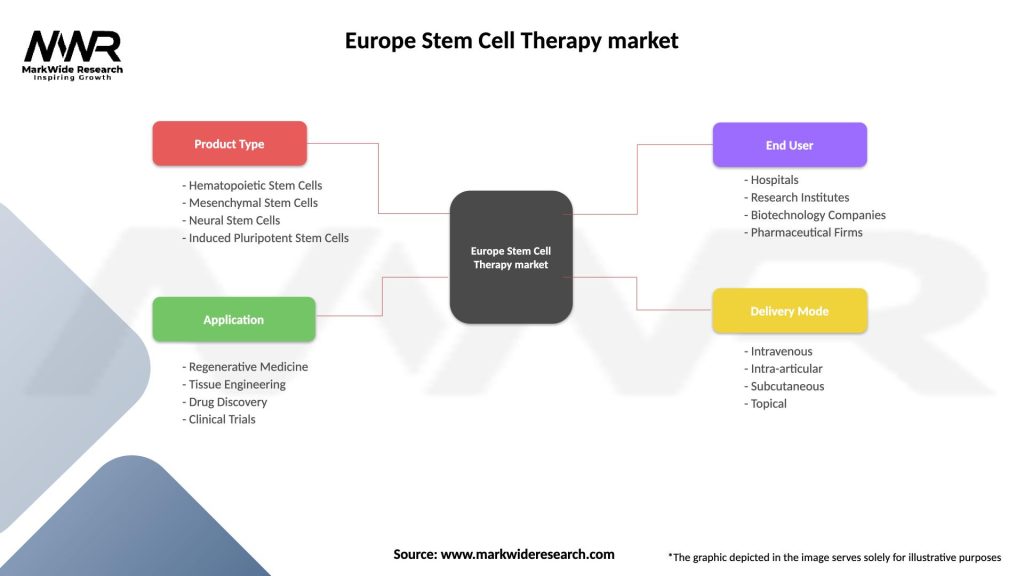
Segmentation
The Europe Stem Cell Therapy Market can be segmented based on:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Key trends shaping the Europe Stem Cell Therapy Market include:
Covid-19 Impact
The COVID-19 pandemic has highlighted the need for advanced therapies, including stem cell treatments, particularly in areas like immune modulation and lung repair. Stem cell therapies are also being explored for their potential to address the long-term effects of COVID-19, including respiratory and neurological complications.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Europe Stem Cell Therapy Market is expected to see continued growth, driven by advances in biotechnology, greater regulatory clarity, and expanding therapeutic applications. As stem cell therapies evolve, they have the potential to revolutionize the treatment of a wide variety of diseases, offering new hope to patients across Europe.
The future outlook for the Europe Stem Cell Therapy market is promising, driven by advancements in regenerative medicine and increasing focus on personalized treatments. Companies that invest in research, collaborations, and patient-centric solutions are likely to thrive in this dynamic market.
Conclusion
The Europe Stem Cell Therapy market is witnessing substantial growth as regenerative medicine becomes a key focus for healthcare providers and researchers. Stem cell therapy offers potential curative treatment options for patients with chronic diseases, presenting an exciting frontier in medical science. The market presents opportunities for biotechnology companies, research institutions, and healthcare providers to pioneer innovative therapies and improve patient outcomes. By addressing ethical considerations, ensuring regulatory compliance, and fostering research collaborations, the stem cell therapy industry can unlock its full potential in transforming healthcare in Europe.
What is Stem Cell Therapy?
Stem Cell Therapy refers to the use of stem cells to treat or prevent diseases and conditions. It involves the transplantation of stem cells to regenerate damaged tissues and organs, with applications in areas such as regenerative medicine and oncology.
What are the key players in the Europe Stem Cell Therapy market?
Key players in the Europe Stem Cell Therapy market include companies like Mesoblast, TiGenix, and Cell Medica, which are involved in developing innovative therapies and conducting clinical trials, among others.
What are the main drivers of the Europe Stem Cell Therapy market?
The main drivers of the Europe Stem Cell Therapy market include the increasing prevalence of chronic diseases, advancements in stem cell research, and growing investments in regenerative medicine. These factors contribute to the rising demand for effective treatment options.
What challenges does the Europe Stem Cell Therapy market face?
The Europe Stem Cell Therapy market faces challenges such as regulatory hurdles, ethical concerns regarding stem cell sourcing, and high costs associated with research and development. These factors can hinder market growth and accessibility.
What opportunities exist in the Europe Stem Cell Therapy market?
Opportunities in the Europe Stem Cell Therapy market include the potential for new treatment modalities, collaborations between research institutions and biotech companies, and the expansion of clinical applications in areas like neurology and cardiology.
What trends are shaping the Europe Stem Cell Therapy market?
Trends shaping the Europe Stem Cell Therapy market include the rise of personalized medicine, advancements in gene editing technologies, and increased focus on cell-based therapies. These trends are driving innovation and expanding treatment possibilities.
Europe Stem Cell Therapy market
| Segmentation Details | Description |
|---|---|
| Product Type | Hematopoietic Stem Cells, Mesenchymal Stem Cells, Neural Stem Cells, Induced Pluripotent Stem Cells |
| Application | Regenerative Medicine, Tissue Engineering, Drug Discovery, Clinical Trials |
| End User | Hospitals, Research Institutes, Biotechnology Companies, Pharmaceutical Firms |
| Delivery Mode | Intravenous, Intra-articular, Subcutaneous, Topical |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Europe Stem Cell Therapy Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at