444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview: The Europe Sickle Cell Disease Treatment Market is witnessing a paradigm shift in the landscape of healthcare as innovative therapies and advancements in treatment options gain prominence. Sickle Cell Disease (SCD) is a genetic blood disorder characterized by abnormal hemoglobin, leading to distorted red blood cells. In Europe, the focus on research and development, coupled with a growing understanding of the genetic basis of SCD, is driving the market toward more effective and personalized treatment approaches.
Meaning: Sickle Cell Disease is a hereditary disorder where the body produces abnormal hemoglobin, causing red blood cells to assume a rigid, sickle shape. This altered shape reduces the cells’ flexibility and longevity, leading to various complications, including pain crises, anemia, and organ damage. The Europe Sickle Cell Disease Treatment Market encompasses a range of therapeutic interventions aimed at alleviating symptoms, preventing complications, and improving the quality of life for individuals affected by SCD.
Executive Summary: The Europe Sickle Cell Disease Treatment Market is experiencing significant growth, propelled by advancements in targeted therapies, gene-based treatments, and a holistic approach to patient care. The market is witnessing increased collaboration between pharmaceutical companies, research institutions, and healthcare providers to develop novel treatments that address the underlying genetic factors contributing to Sickle Cell Disease. This collaborative effort is reshaping the treatment landscape and offering new hope to patients across Europe.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The dynamics of the Europe Sickle Cell Disease Treatment Market are shaped by a combination of scientific advancements, healthcare policies, patient advocacy, and socioeconomic factors. The market operates in a dynamic environment where ongoing research, regulatory developments, and technological innovations influence the landscape.
Regional Analysis: The prevalence of Sickle Cell Disease and the landscape of its treatment vary across European regions. While some countries have well-established healthcare infrastructure and comprehensive SCD programs, others face challenges related to awareness, access to healthcare, and the availability of specialized treatments.
Competitive Landscape:
Leading Companies in the Europe Sickle Cell Disease Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
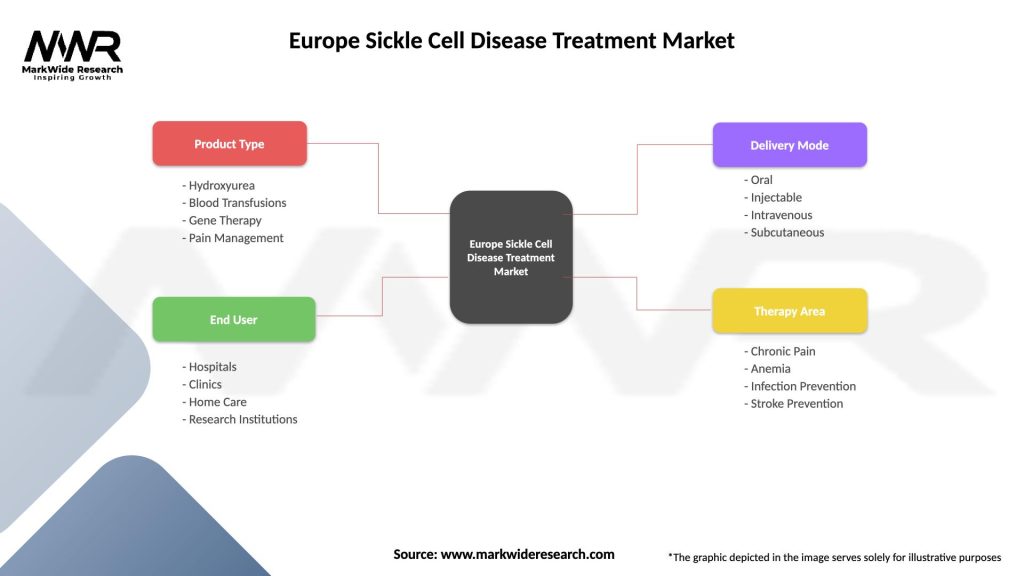
Segmentation: The Europe Sickle Cell Disease Treatment Market can be segmented based on various factors:
Segmentation provides a detailed understanding of the market dynamics, allowing stakeholders to tailor interventions and strategies based on specific regional and demographic factors.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis: A SWOT analysis provides insights into the Europe Sickle Cell Disease Treatment Market’s strengths, weaknesses, opportunities, and threats:
Strengths:
Weaknesses:
Opportunities:
Threats:
Understanding these factors through a SWOT analysis enables industry participants to strategically navigate challenges, capitalize on opportunities, and contribute to the growth of the market.
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has introduced both challenges and opportunities in the Europe Sickle Cell Disease Treatment Market. Disruptions in healthcare services, supply chains, and research activities were observed during the initial phase of the pandemic. However, the increased focus on telemedicine, remote monitoring, and digital health solutions has provided a platform for innovation in patient care and disease management.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The Europe Sickle Cell Disease Treatment Market is poised for transformative growth, driven by advancements in gene therapies, personalized medicine, and collaborative research initiatives. The future outlook suggests a shift toward curative approaches, improved patient outcomes, and a more holistic understanding of Sickle Cell Disease as a genetic disorder.
Conclusion: As the Europe Sickle Cell Disease Treatment Market evolves, it reflects a dynamic landscape marked by scientific breakthroughs, patient-centered care models, and collaborative efforts to address the unique challenges posed by Sickle Cell Disease. The convergence of gene therapies, pharmacotherapies, and innovative treatment strategies positions the market for continued growth and improved outcomes for individuals affected by Sickle Cell Disease in the region. By embracing advancements, fostering partnerships, and prioritizing patient needs, the industry can contribute to a future where Sickle Cell Disease is effectively managed, and individuals lead healthier lives.
What is Sickle Cell Disease Treatment?
Sickle Cell Disease Treatment refers to the medical approaches used to manage and alleviate the symptoms of sickle cell disease, a genetic blood disorder characterized by the production of abnormal hemoglobin. Treatments may include pain management, blood transfusions, and medications aimed at reducing complications.
What are the key players in the Europe Sickle Cell Disease Treatment Market?
Key players in the Europe Sickle Cell Disease Treatment Market include Novartis, Pfizer, and Bluebird Bio, which are involved in developing innovative therapies and treatments for sickle cell disease, among others.
What are the growth factors driving the Europe Sickle Cell Disease Treatment Market?
The Europe Sickle Cell Disease Treatment Market is driven by factors such as increasing awareness of sickle cell disease, advancements in treatment options, and a growing patient population requiring effective management solutions.
What challenges does the Europe Sickle Cell Disease Treatment Market face?
Challenges in the Europe Sickle Cell Disease Treatment Market include high treatment costs, limited access to specialized care, and the need for more comprehensive healthcare policies to support affected individuals.
What opportunities exist in the Europe Sickle Cell Disease Treatment Market?
Opportunities in the Europe Sickle Cell Disease Treatment Market include the development of gene therapies, increased investment in research and development, and potential collaborations between pharmaceutical companies and healthcare providers to enhance treatment accessibility.
What trends are shaping the Europe Sickle Cell Disease Treatment Market?
Trends in the Europe Sickle Cell Disease Treatment Market include the rise of personalized medicine, the integration of digital health technologies for patient monitoring, and a focus on holistic care approaches that address both physical and psychological aspects of the disease.
Europe Sickle Cell Disease Treatment Market
| Segmentation Details | Description |
|---|---|
| Product Type | Hydroxyurea, Blood Transfusions, Gene Therapy, Pain Management |
| End User | Hospitals, Clinics, Home Care, Research Institutions |
| Delivery Mode | Oral, Injectable, Intravenous, Subcutaneous |
| Therapy Area | Chronic Pain, Anemia, Infection Prevention, Stroke Prevention |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Europe Sickle Cell Disease Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at