444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview: The Europe mRNA vaccines and RNAi therapeutics market is at the forefront of innovative medical interventions, revolutionizing the landscape of preventive and therapeutic healthcare. The market’s significant growth is driven by advancements in biotechnology, increasing awareness of personalized medicine, and the successful deployment of mRNA vaccines in addressing infectious diseases. This comprehensive overview delves into the meaning, executive summary, key market insights, drivers, restraints, opportunities, market dynamics, regional analysis, competitive landscape, segmentation, category-wise insights, key benefits for industry participants, SWOT analysis, market key trends, COVID-19 impact, key industry developments, analyst suggestions, future outlook, and conclusion of the Europe mRNA vaccines and RNAi therapeutics market.
Meaning: mRNA vaccines and RNAi therapeutics represent cutting-edge biotechnological approaches designed to harness the body’s own cellular machinery for therapeutic purposes. mRNA vaccines stimulate an immune response by introducing genetic material that encodes specific viral or pathogenic proteins, training the immune system to recognize and combat potential threats. RNAi therapeutics leverage RNA interference to selectively silence or modulate the expression of target genes, offering a promising avenue for treating various diseases at the genetic level.
Executive Summary: The Europe mRNA vaccines and RNAi therapeutics market is witnessing remarkable growth, fueled by advancements in biotechnology, rising investments in research and development, and the successful deployment of mRNA vaccines for infectious diseases. This market presents lucrative opportunities for industry participants but is not without its challenges, including regulatory complexities and the need for substantial investments in technology and infrastructure.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics: The Europe mRNA vaccines and RNAi therapeutics market operates in a dynamic environment influenced by factors such as technological advancements, regulatory developments, market trends, and global health crises. Stakeholders need to adapt and innovate to navigate the evolving landscape, capitalize on opportunities, and address challenges effectively.
Regional Analysis:
Competitive Landscape:
Leading Companies in the Europe mRNA Vaccines and RNAi Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
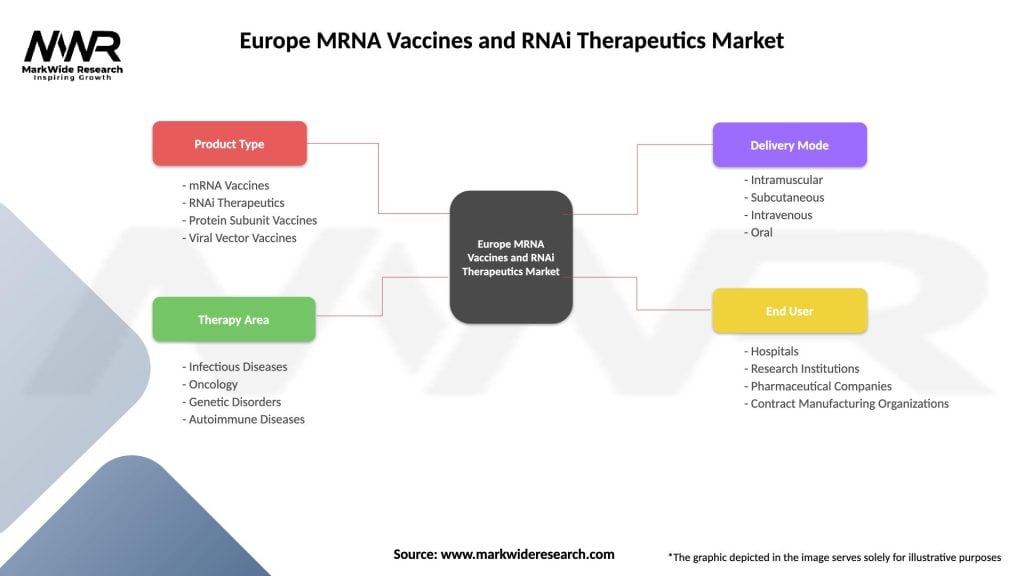
Segmentation: The market can be segmented based on therapeutic applications, including infectious diseases, oncology, genetic disorders, and rare diseases. Additionally, segmentation by technology and delivery systems, such as lipid nanoparticles and viral vectors, provides a detailed understanding of the market dynamics.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis: A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats of the Europe mRNA vaccines and RNAi therapeutics market.
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
COVID-19 Impact: The COVID-19 pandemic has accelerated the adoption of mRNA vaccine technologies, with successful deployment against the SARS-CoV-2 virus. The pandemic highlighted the agility of mRNA platforms in responding to emerging threats and underscored the potential of RNAi therapeutics in addressing viral infections.
Key Industry Developments:
Analyst Suggestions:
Future Outlook: The future outlook for the Europe mRNA vaccines and RNAi therapeutics market is optimistic, with continued advancements expected in technology, expanded therapeutic applications, and increased collaborations. The market is poised for sustained growth, driven by a commitment to addressing unmet medical needs, leveraging innovative approaches, and contributing to the evolution of healthcare.
Conclusion: The Europe mRNA vaccines and RNAi therapeutics market stands at the forefront of transformative healthcare solutions, offering unprecedented opportunities to address infectious diseases, oncology, and genetic disorders. While facing regulatory complexities and technological challenges, the market benefits from a collaborative ecosystem, innovative advancements, and increasing investments. As the region continues to lead in innovation and research, the future of mRNA and RNAi therapeutics in Europe holds promise for revolutionizing healthcare practices, advancing personalized medicine, and contributing to the global progress of biotechnological interventions. Industry stakeholders are poised to play a pivotal role in shaping this future by navigating challenges, embracing innovation, and delivering impactful solutions to improve patient outcomes.
What is MRNA Vaccines and RNAi Therapeutics?
MRNA Vaccines and RNAi Therapeutics refer to innovative medical technologies that utilize messenger RNA to instruct cells to produce proteins that can prevent or treat diseases, including viral infections and genetic disorders.
What are the key players in the Europe MRNA Vaccines and RNAi Therapeutics Market?
Key players in the Europe MRNA Vaccines and RNAi Therapeutics Market include BioNTech, Moderna, and CureVac, which are known for their advancements in mRNA technology and vaccine development, among others.
What are the growth factors driving the Europe MRNA Vaccines and RNAi Therapeutics Market?
The growth of the Europe MRNA Vaccines and RNAi Therapeutics Market is driven by increasing investments in biotechnology, rising demand for personalized medicine, and the urgent need for effective vaccines against emerging infectious diseases.
What challenges does the Europe MRNA Vaccines and RNAi Therapeutics Market face?
Challenges in the Europe MRNA Vaccines and RNAi Therapeutics Market include regulatory hurdles, public skepticism regarding vaccine safety, and the complexity of manufacturing mRNA products at scale.
What future opportunities exist in the Europe MRNA Vaccines and RNAi Therapeutics Market?
Future opportunities in the Europe MRNA Vaccines and RNAi Therapeutics Market include the development of mRNA-based therapies for cancer treatment, advancements in delivery mechanisms, and potential applications in rare genetic disorders.
What trends are shaping the Europe MRNA Vaccines and RNAi Therapeutics Market?
Trends shaping the Europe MRNA Vaccines and RNAi Therapeutics Market include the increasing collaboration between pharmaceutical companies and academic institutions, the rise of digital health technologies, and a focus on sustainable manufacturing practices.
Europe MRNA Vaccines and RNAi Therapeutics Market
| Segmentation Details | Description |
|---|---|
| Product Type | mRNA Vaccines, RNAi Therapeutics, Protein Subunit Vaccines, Viral Vector Vaccines |
| Therapy Area | Infectious Diseases, Oncology, Genetic Disorders, Autoimmune Diseases |
| Delivery Mode | Intramuscular, Subcutaneous, Intravenous, Oral |
| End User | Hospitals, Research Institutions, Pharmaceutical Companies, Contract Manufacturing Organizations |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Europe mRNA Vaccines and RNAi Therapeutics Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at