444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
The Europe monoclonal antibodies market represents one of the most dynamic and rapidly evolving segments within the global biopharmaceutical industry. Monoclonal antibodies have revolutionized therapeutic approaches across multiple disease areas, establishing themselves as cornerstone treatments for cancer, autoimmune disorders, and inflammatory conditions. The European market demonstrates exceptional growth momentum, driven by increasing prevalence of chronic diseases, advancing biotechnology capabilities, and robust regulatory frameworks that support innovation.
Market dynamics in Europe reflect a sophisticated healthcare ecosystem where cutting-edge research meets clinical excellence. The region benefits from strong pharmaceutical infrastructure, world-class research institutions, and collaborative networks between academia and industry. Growth rates consistently outpace global averages, with the market expanding at a compound annual growth rate of 8.2% through the forecast period.
European countries including Germany, France, the United Kingdom, and Switzerland lead market development through substantial investments in biotechnology research and development. These nations host major pharmaceutical companies and biotechnology firms that drive innovation in monoclonal antibody development, manufacturing, and commercialization.
The Europe monoclonal antibodies market refers to the comprehensive ecosystem encompassing research, development, manufacturing, and commercialization of monoclonal antibody therapeutics across European territories. Monoclonal antibodies are laboratory-produced molecules engineered to serve as substitute antibodies that can restore, enhance, or mimic the immune system’s attack on cells, particularly targeting specific antigens associated with diseases.
These therapeutic proteins represent a class of biologics designed to bind to specific targets with high precision, offering targeted treatment approaches that minimize side effects while maximizing therapeutic efficacy. The European market encompasses various monoclonal antibody types, including fully human, humanized, chimeric, and murine antibodies, each designed for specific therapeutic applications.
Market scope includes therapeutic areas such as oncology, immunology, neurology, cardiovascular diseases, and infectious diseases. The definition extends beyond finished pharmaceutical products to include contract research organizations, manufacturing services, and supporting technologies that enable monoclonal antibody development and production throughout Europe.
Europe’s monoclonal antibodies market stands at the forefront of global biopharmaceutical innovation, characterized by robust growth, technological advancement, and expanding therapeutic applications. The market benefits from strong regulatory support through the European Medicines Agency, facilitating faster approval processes for breakthrough therapies while maintaining rigorous safety standards.
Key growth drivers include rising cancer incidence rates, increasing prevalence of autoimmune diseases, and growing adoption of personalized medicine approaches. Oncology applications dominate market share, representing approximately 45% of total monoclonal antibody utilization across European healthcare systems. The immunology segment follows closely, driven by treatments for rheumatoid arthritis, inflammatory bowel disease, and psoriasis.
Manufacturing capabilities across Europe continue expanding, with significant investments in biomanufacturing facilities and contract manufacturing organizations. The region’s emphasis on biosimilar development creates additional market opportunities while promoting cost-effective treatment access. Research and development activities remain intensive, with European pharmaceutical companies investing heavily in next-generation monoclonal antibody technologies including antibody-drug conjugates and bispecific antibodies.
Market challenges include pricing pressures from healthcare systems, complex regulatory requirements, and increasing competition from biosimilar products. However, continuous innovation and expanding therapeutic applications maintain strong growth prospects for the European monoclonal antibodies market.

Strategic market insights reveal several critical trends shaping the European monoclonal antibodies landscape. The market demonstrates remarkable resilience and growth potential across multiple dimensions:
Market penetration rates vary significantly across European countries, with Western European nations showing higher adoption rates compared to Eastern European markets. Germany leads in both market size and innovation, followed by France and the United Kingdom, while emerging markets in Eastern Europe present substantial growth opportunities.
Primary market drivers propelling the European monoclonal antibodies market include several interconnected factors that create sustained demand growth. Demographic trends represent the most significant driver, with Europe’s aging population experiencing higher incidence rates of cancer, autoimmune diseases, and chronic conditions that benefit from monoclonal antibody treatments.
Cancer prevalence continues rising across European populations, with monoclonal antibodies providing targeted therapeutic options that improve patient outcomes while reducing treatment-related side effects. Oncology applications drive approximately 60% of market growth, supported by continuous development of novel cancer-targeting antibodies and combination therapy approaches.
Autoimmune disease management represents another crucial driver, with conditions like rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease affecting millions of Europeans. Monoclonal antibodies offer disease-modifying treatments that significantly improve quality of life and long-term prognosis for patients with these conditions.
Healthcare system evolution toward value-based care models encourages adoption of effective biologics that demonstrate clear clinical benefits and economic value. Reimbursement policies increasingly favor treatments that provide superior outcomes, supporting monoclonal antibody utilization across therapeutic areas.
Technological advancement in antibody engineering enables development of more effective and safer therapeutic options. Next-generation antibodies including bispecific antibodies, antibody-drug conjugates, and immune checkpoint inhibitors expand treatment possibilities and market opportunities.
Market restraints present significant challenges that could potentially limit growth in the European monoclonal antibodies market. High development costs associated with monoclonal antibody research, clinical trials, and regulatory approval create substantial barriers for smaller biotechnology companies and limit the number of new market entrants.
Pricing pressures from European healthcare systems increasingly challenge pharmaceutical companies to demonstrate value and cost-effectiveness. Health technology assessment processes require comprehensive economic evaluations that may delay market access or result in restricted reimbursement coverage for new monoclonal antibody therapies.
Manufacturing complexity associated with biologics production requires specialized facilities, skilled personnel, and stringent quality control measures. These requirements create significant capital investment needs and operational challenges that may limit manufacturing capacity and increase production costs.
Regulatory complexity across different European countries creates additional challenges for market access and commercialization. While the European Medicines Agency provides centralized approval, individual country-level reimbursement decisions and pricing negotiations can significantly impact market penetration timelines.
Biosimilar competition intensifies as patents expire on established monoclonal antibody products. Price erosion from biosimilar entry affects revenue potential for originator products and may reduce incentives for continued innovation investment.
Safety concerns and potential adverse events associated with monoclonal antibody treatments require careful monitoring and may limit prescribing in certain patient populations. Immunogenicity risks and long-term safety profiles continue requiring extensive post-market surveillance and research.
Emerging opportunities in the European monoclonal antibodies market present substantial potential for growth and innovation. Rare disease applications offer significant opportunities as European regulatory agencies provide incentives for orphan drug development, including extended market exclusivity and reduced regulatory fees.
Combination therapy approaches combining monoclonal antibodies with other treatment modalities create new therapeutic possibilities and market segments. Immuno-oncology combinations particularly show promise, with multiple antibodies targeting different pathways to enhance treatment efficacy and overcome resistance mechanisms.
Emerging markets in Eastern Europe present substantial growth opportunities as healthcare systems modernize and access to advanced biologics improves. Market expansion in countries like Poland, Czech Republic, and Hungary offers significant potential for companies establishing early market presence.
Digital health integration creates opportunities for companion diagnostics, patient monitoring systems, and personalized treatment approaches. Biomarker development enables more precise patient selection and treatment optimization, potentially improving outcomes while reducing healthcare costs.
Manufacturing innovation including continuous manufacturing, single-use technologies, and automated production systems offer opportunities to reduce costs and improve supply chain efficiency. Contract manufacturing services continue expanding to meet growing demand from biotechnology companies.
Next-generation antibody formats including antibody fragments, bispecific antibodies, and antibody-drug conjugates represent significant innovation opportunities that could capture substantial market share in coming years.
Market dynamics in the European monoclonal antibodies sector reflect complex interactions between innovation, regulation, competition, and healthcare economics. Supply and demand patterns show strong demand growth outpacing supply capacity in certain therapeutic areas, creating opportunities for manufacturing expansion and new market entrants.
Competitive dynamics intensify as both established pharmaceutical companies and emerging biotechnology firms compete for market share. Innovation cycles accelerate with companies investing heavily in research and development to maintain competitive advantages and develop next-generation therapeutic options.
Pricing dynamics evolve as healthcare systems implement value-based pricing models and negotiate more aggressively for cost-effective treatments. Market access strategies become increasingly important as companies must demonstrate not only clinical efficacy but also economic value and budget impact.
Regulatory dynamics continue evolving with agencies adapting to new technologies and treatment approaches. Adaptive trial designs and accelerated approval pathways enable faster market access for breakthrough therapies while maintaining safety standards.
Technology dynamics drive continuous innovation in antibody discovery, engineering, and manufacturing. Artificial intelligence and machine learning applications increasingly support drug discovery and development processes, potentially reducing timelines and improving success rates.
Partnership dynamics show increasing collaboration between pharmaceutical companies, biotechnology firms, and academic institutions. Strategic alliances enable risk sharing, resource optimization, and access to complementary capabilities across the value chain.
Research methodology for analyzing the European monoclonal antibodies market employs comprehensive primary and secondary research approaches to ensure data accuracy and market insight reliability. Primary research includes extensive interviews with key industry stakeholders including pharmaceutical executives, biotechnology company leaders, healthcare providers, and regulatory experts across major European markets.
Secondary research encompasses analysis of published clinical trial data, regulatory filings, company financial reports, and industry publications. Data triangulation methods validate findings across multiple sources to ensure consistency and reliability of market insights and projections.
Market sizing methodology utilizes bottom-up and top-down approaches, analyzing therapeutic area segments, geographic markets, and competitive landscapes. Growth projections incorporate multiple scenarios considering various market drivers, restraints, and emerging trends that could impact future market development.
Quantitative analysis includes statistical modeling of market trends, growth rates, and competitive dynamics. Qualitative analysis provides context for market developments, regulatory changes, and strategic implications for industry participants.
Expert validation processes involve review of findings with industry experts and key opinion leaders to ensure accuracy and relevance of market insights. Continuous monitoring of market developments ensures research findings remain current and actionable for stakeholders.
Regional analysis reveals significant variations in monoclonal antibodies market development across European territories. Western Europe dominates market activity, accounting for approximately 75% of total European market share, with Germany, France, and the United Kingdom leading in both market size and innovation activities.
Germany represents the largest European market for monoclonal antibodies, driven by robust healthcare infrastructure, strong pharmaceutical industry presence, and supportive reimbursement policies. German biotechnology companies contribute significantly to global monoclonal antibody development, with several major manufacturing facilities supporting both domestic and international markets.
France demonstrates strong market growth supported by government initiatives promoting biotechnology innovation and healthcare modernization. French pharmaceutical companies maintain significant research and development activities in monoclonal antibody development, particularly in oncology and immunology applications.
United Kingdom continues playing a crucial role despite Brexit-related uncertainties, with London remaining a major biotechnology hub and several leading pharmaceutical companies maintaining substantial operations. UK regulatory frameworks continue aligning with European standards while developing independent capabilities.
Eastern Europe presents substantial growth opportunities with markets like Poland, Czech Republic, and Hungary showing rapid adoption rates. Healthcare modernization initiatives and improving reimbursement coverage drive market expansion in these regions, though growth rates vary significantly between countries.
Nordic countries including Sweden, Denmark, and Norway demonstrate high per-capita utilization rates and strong support for innovative therapies. Scandinavian healthcare systems often serve as early adopters for new monoclonal antibody treatments, providing valuable real-world evidence for broader European adoption.
Competitive landscape in the European monoclonal antibodies market features a diverse mix of multinational pharmaceutical companies, specialized biotechnology firms, and emerging players developing innovative therapeutic approaches. Market leadership positions shift dynamically as companies launch new products and expand therapeutic applications.
Competitive strategies focus on innovation, strategic partnerships, and market access optimization. Research and development investments remain high as companies compete to develop next-generation antibody formats and expand into new therapeutic areas.
Biosimilar competition intensifies as patents expire on major monoclonal antibody products. European biosimilar companies including Sandoz, Teva, and Celltrion gain market share through competitive pricing and robust regulatory approval strategies.
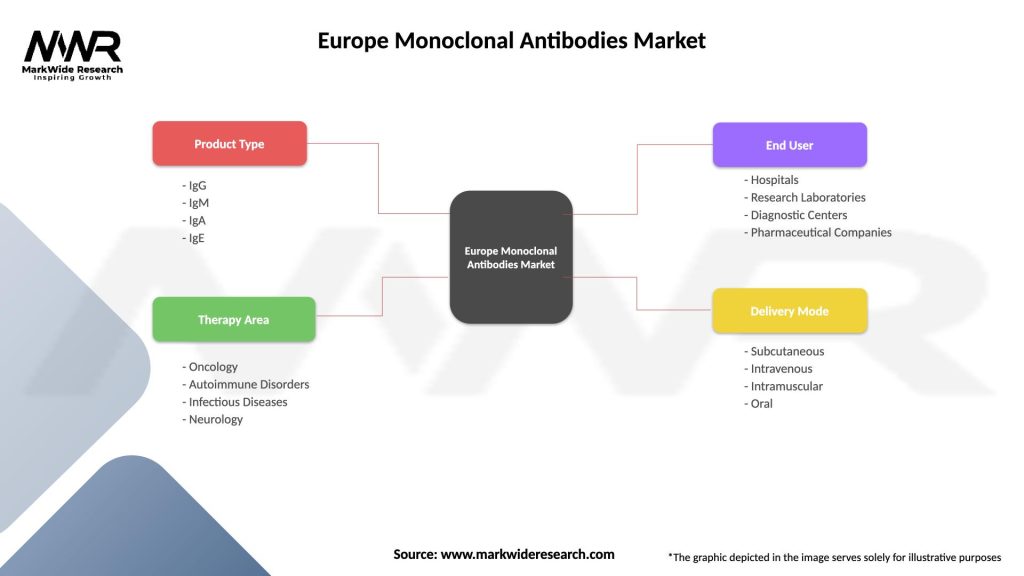
Market segmentation analysis reveals distinct patterns across multiple dimensions including therapeutic application, antibody type, end-user, and geographic distribution. Therapeutic segmentation shows oncology applications maintaining dominant market share while immunology and neurology segments demonstrate rapid growth rates.
By Therapeutic Application:
By Antibody Type:
By End User:
Category-wise analysis provides detailed insights into specific market segments and their unique characteristics, growth drivers, and competitive dynamics. Oncology monoclonal antibodies represent the most mature and largest category, with established treatment protocols and continuous innovation in targeted therapy approaches.
Immuno-oncology subcategory shows exceptional growth with checkpoint inhibitors revolutionizing cancer treatment paradigms. PD-1/PD-L1 inhibitors demonstrate particular success across multiple cancer types, with combination therapy approaches expanding treatment possibilities and improving patient outcomes.
Immunology category benefits from expanding understanding of autoimmune disease mechanisms and development of targeted therapeutic approaches. TNF-alpha inhibitors maintain strong market positions while newer targets including IL-17, IL-23, and JAK pathways create additional growth opportunities.
Neurology applications represent an emerging high-growth category with monoclonal antibodies targeting neuroinflammation, amyloid plaques, and other neurological disease mechanisms. Multiple sclerosis treatments show particular promise with several new approvals expanding treatment options.
Rare disease applications benefit from regulatory incentives and premium pricing, creating attractive opportunities for specialized biotechnology companies. Orphan drug designations provide market exclusivity and development support that encourage innovation in underserved patient populations.
Biosimilar category grows rapidly as patents expire on major reference products. European biosimilar adoption rates exceed global averages, with healthcare systems actively promoting cost-effective alternatives to maintain treatment access while managing budget constraints.
Industry participants and stakeholders realize substantial benefits from the expanding European monoclonal antibodies market across multiple dimensions. Pharmaceutical companies benefit from strong revenue growth potential, premium pricing opportunities, and extended patent protection periods that support return on investment for research and development activities.
Biotechnology companies gain access to sophisticated European markets with supportive regulatory frameworks and established reimbursement systems. Partnership opportunities with established pharmaceutical companies provide access to manufacturing capabilities, distribution networks, and regulatory expertise necessary for successful commercialization.
Healthcare providers benefit from expanded treatment options that improve patient outcomes while potentially reducing long-term healthcare costs through more effective disease management. Targeted therapies enable personalized treatment approaches that optimize efficacy while minimizing adverse events.
Patients realize the most significant benefits through access to innovative treatments that improve quality of life, extend survival, and provide hope for previously untreatable conditions. Treatment advances in oncology, immunology, and rare diseases directly translate to improved patient outcomes and reduced disease burden.
Healthcare systems benefit from value-based treatment options that demonstrate clear clinical and economic benefits. Cost-effectiveness of monoclonal antibody treatments often justifies premium pricing through reduced hospitalization rates, improved productivity, and better long-term outcomes.
Research institutions benefit from collaborative opportunities with industry partners, access to cutting-edge technologies, and funding for basic research that advances scientific understanding of disease mechanisms and therapeutic approaches.
Strengths:
Weaknesses:
Opportunities:
Threats:
Key market trends shaping the European monoclonal antibodies landscape reflect technological advancement, changing healthcare needs, and evolving regulatory environments. Personalized medicine emerges as a dominant trend, with increasing focus on biomarker-driven treatment selection and companion diagnostic development.
Next-generation antibody formats gain momentum with bispecific antibodies, antibody-drug conjugates, and immune cell engagers showing superior efficacy profiles compared to conventional monoclonal antibodies. Technology advancement enables more sophisticated targeting mechanisms and improved therapeutic indices.
Combination therapy approaches become standard practice across multiple therapeutic areas, particularly in oncology where immune checkpoint inhibitors combine with targeted therapies to enhance treatment outcomes. Rational combinations based on mechanistic understanding drive development strategies and clinical trial designs.
Manufacturing innovation trends include adoption of continuous manufacturing processes, single-use technologies, and automated production systems. Cost reduction initiatives focus on improving manufacturing efficiency while maintaining quality standards required for biologics production.
Digital health integration accelerates with artificial intelligence supporting drug discovery, patient monitoring systems enabling remote care, and real-world evidence generation informing treatment decisions. Data analytics capabilities enhance understanding of treatment patterns and outcomes.
Sustainability initiatives gain importance as companies implement environmentally responsible manufacturing practices and develop more efficient production processes. Green biotechnology approaches reduce environmental impact while maintaining product quality and efficacy.
Recent industry developments highlight the dynamic nature of the European monoclonal antibodies market with significant advances in research, regulatory approvals, and commercial activities. Regulatory approvals for breakthrough therapies continue accelerating, with several novel monoclonal antibodies receiving European Medicines Agency approval for previously untreatable conditions.
Strategic partnerships between pharmaceutical companies and biotechnology firms intensify as organizations seek to combine complementary capabilities and share development risks. Collaboration agreements increasingly focus on next-generation antibody formats and novel therapeutic targets.
Manufacturing investments expand across Europe with companies establishing new biomanufacturing facilities and upgrading existing production capabilities. Capacity expansion projects address growing demand while implementing advanced technologies that improve efficiency and reduce costs.
Biosimilar launches continue as patents expire on major reference products, with European companies leading global biosimilar development and commercialization efforts. Market competition intensifies as multiple biosimilar versions become available for established monoclonal antibody treatments.
Digital transformation initiatives accelerate across the industry with companies implementing artificial intelligence in drug discovery, adopting digital clinical trial approaches, and developing patient-centric digital health solutions. Technology integration enhances efficiency and improves patient outcomes.
Regulatory harmonization efforts continue with European agencies working to streamline approval processes while maintaining safety standards. Adaptive pathways and accelerated approval mechanisms enable faster access to breakthrough therapies for patients with high unmet medical needs.
Strategic recommendations for stakeholders in the European monoclonal antibodies market focus on positioning for long-term success in an increasingly competitive and dynamic environment. MarkWide Research analysis suggests that companies should prioritize innovation in next-generation antibody formats while building robust manufacturing capabilities to support growth.
Investment priorities should emphasize research and development in emerging therapeutic areas including neurology, rare diseases, and infectious diseases where significant unmet medical needs exist. Portfolio diversification across multiple therapeutic areas reduces risk while capturing growth opportunities in expanding market segments.
Market access strategies require early engagement with health technology assessment bodies and payers to demonstrate value propositions and secure favorable reimbursement decisions. Real-world evidence generation becomes increasingly important for supporting market access and maintaining competitive positions.
Partnership strategies should focus on complementary capabilities including specialized research expertise, manufacturing capacity, and geographic market access. Strategic alliances enable risk sharing while accelerating development timelines and market penetration.
Manufacturing optimization initiatives should emphasize continuous improvement, cost reduction, and capacity expansion to meet growing demand. Technology adoption including single-use systems and automated processes improves efficiency while maintaining quality standards.
Digital transformation investments should focus on artificial intelligence applications in drug discovery, patient monitoring systems, and data analytics capabilities that enhance decision-making and improve outcomes. Technology integration provides competitive advantages in increasingly sophisticated markets.
Future outlook for the European monoclonal antibodies market remains exceptionally positive, with sustained growth expected across multiple dimensions through the forecast period. Market expansion will be driven by continued innovation, expanding therapeutic applications, and growing adoption in emerging European markets.
Growth projections indicate the market will maintain robust expansion rates, with compound annual growth expected to reach 8.5% through 2030. Innovation cycles will accelerate as companies invest heavily in next-generation antibody formats and novel therapeutic targets that address previously untreatable conditions.
Therapeutic expansion will continue with monoclonal antibodies entering new disease areas including neurological disorders, cardiovascular diseases, and metabolic conditions. Precision medicine approaches will become standard practice, with biomarker-driven treatment selection optimizing patient outcomes while improving cost-effectiveness.
Technology advancement will enable more sophisticated antibody engineering approaches, improved manufacturing processes, and enhanced patient monitoring capabilities. Artificial intelligence integration will accelerate drug discovery timelines while improving success rates and reducing development costs.
Market access will evolve toward value-based pricing models that reward treatments demonstrating superior clinical and economic outcomes. Reimbursement frameworks will increasingly incorporate real-world evidence and patient-reported outcomes in coverage decisions.
Competitive dynamics will intensify as biosimilar competition expands while innovation in novel antibody formats creates new market opportunities. MWR projections suggest successful companies will balance innovation investment with operational efficiency to maintain competitive positions in evolving markets.
The Europe monoclonal antibodies market represents one of the most dynamic and promising segments within the global biopharmaceutical industry, characterized by robust growth, continuous innovation, and expanding therapeutic applications. Market fundamentals remain strong with increasing disease prevalence, advancing technology, and supportive regulatory frameworks driving sustained demand growth.
Strategic opportunities abound for companies positioned to capitalize on emerging trends including next-generation antibody formats, personalized medicine approaches, and expansion into underserved therapeutic areas. Innovation leadership will determine competitive success as the market evolves toward more sophisticated and targeted treatment approaches.
Challenges including pricing pressures, regulatory complexity, and biosimilar competition require strategic responses that balance innovation investment with operational efficiency. Successful market participants will demonstrate agility in adapting to changing market dynamics while maintaining focus on patient outcomes and value creation.
Future prospects remain exceptionally positive with the Europe monoclonal antibodies market expected to continue its trajectory of strong growth and innovation leadership. Stakeholders who invest strategically in research and development, manufacturing capabilities, and market access will be well-positioned to capture the substantial opportunities ahead in this transformative healthcare sector.
What is Monoclonal Antibodies?
Monoclonal antibodies are laboratory-made molecules that can mimic the immune system’s ability to fight off harmful pathogens such as viruses. They are used in various applications, including cancer treatment, autoimmune diseases, and infectious diseases.
What are the key players in the Europe Monoclonal Antibodies Market?
Key players in the Europe Monoclonal Antibodies Market include Roche, AbbVie, and Merck, which are known for their innovative therapies and extensive research in monoclonal antibody development, among others.
What are the drivers of growth in the Europe Monoclonal Antibodies Market?
The growth of the Europe Monoclonal Antibodies Market is driven by increasing prevalence of chronic diseases, advancements in biotechnology, and rising investments in research and development for new therapeutic applications.
What challenges does the Europe Monoclonal Antibodies Market face?
Challenges in the Europe Monoclonal Antibodies Market include high development costs, stringent regulatory requirements, and potential competition from biosimilars, which can impact market dynamics.
What opportunities exist in the Europe Monoclonal Antibodies Market?
Opportunities in the Europe Monoclonal Antibodies Market include the development of personalized medicine, expansion into emerging markets, and the potential for novel applications in treating various diseases.
What trends are shaping the Europe Monoclonal Antibodies Market?
Trends in the Europe Monoclonal Antibodies Market include the increasing use of combination therapies, advancements in antibody engineering, and a growing focus on immunotherapy as a treatment option.
Europe Monoclonal Antibodies Market
| Segmentation Details | Description |
|---|---|
| Product Type | IgG, IgM, IgA, IgE |
| Therapy Area | Oncology, Autoimmune Disorders, Infectious Diseases, Neurology |
| End User | Hospitals, Research Laboratories, Diagnostic Centers, Pharmaceutical Companies |
| Delivery Mode | Subcutaneous, Intravenous, Intramuscular, Oral |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Europe Monoclonal Antibodies Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at