444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
The Europe medical device reprocessing market is experiencing strong growth as healthcare providers increasingly adopt cost-effective and sustainable solutions for managing single-use medical devices. Reprocessing involves the cleaning, sterilization, functional testing, and repackaging of medical devices to ensure they can be safely reused. The practice reduces healthcare costs, minimizes waste, and supports sustainability initiatives, which are becoming a priority across European healthcare systems. The market is expanding at a CAGR of over 10.2%, reflecting regulatory support, growing hospital adoption, and increasing pressure to optimize healthcare budgets.
Reprocessed devices are widely used in cardiology, orthopedic, gastroenterology, and general surgical procedures. In Europe, over 45% of hospitals now use reprocessed devices for selected applications, up from just 30% five years ago. The rising focus on green healthcare, coupled with high costs associated with disposable medical devices, has positioned reprocessing as a vital strategy for both economic and environmental sustainability. According to MarkWide Research, the market is set to expand significantly as regulatory harmonization and technological advancements continue to strengthen confidence in the safety and efficacy of reprocessed devices.
The medical device reprocessing market refers to the industry dedicated to extending the usable life of medical devices through regulated cleaning, sterilization, and functional verification processes. This includes single-use devices such as catheters, forceps, surgical instruments, and electrophysiology devices that are collected after use, decontaminated, and returned to healthcare providers for safe reuse. Reprocessing is conducted under strict European Union Medical Device Regulation (EU MDR) standards, ensuring compliance with safety and quality benchmarks.
Reprocessing offers significant benefits, including cost savings of up to 50% per device and reductions in medical waste by as much as 25–30%. MWR highlights that the practice is not only an economic strategy but also a sustainability imperative, aligning with Europe’s climate goals and the healthcare sector’s commitment to environmental responsibility.
The Europe medical device reprocessing market is rapidly gaining momentum as hospitals and clinics embrace sustainable procurement practices. Driven by the dual imperatives of cost efficiency and environmental stewardship, the market is projected to grow at a 10.2% CAGR over the forecast period. Reprocessed devices are increasingly accepted for use in cardiology and electrophysiology procedures, where they deliver comparable safety and performance to new devices at lower costs.
Market expansion is supported by favorable EU regulations, growing acceptance by healthcare providers, and technological advances in sterilization and tracking. With over 60% of hospitals in Western Europe considering reprocessing programs, adoption is set to grow further. According to MarkWide Research, strategic collaborations between hospitals, third-party reprocessors, and manufacturers will shape the future landscape, ensuring both quality assurance and widespread adoption.
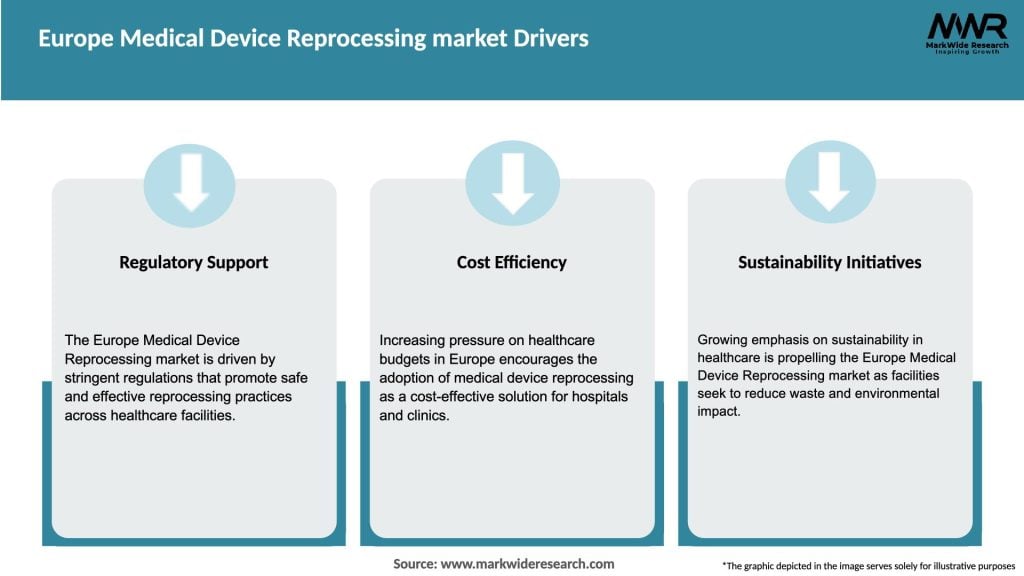
Cost containment in healthcare is a primary driver, as reprocessed devices provide hospitals with significant savings compared to purchasing new devices. Growing environmental awareness also drives demand, with healthcare systems under pressure to reduce their carbon footprint. Reprocessing supports green initiatives by reducing medical waste volumes and reliance on disposable devices.
Additionally, regulatory support under EU MDR has strengthened confidence in the quality and safety of reprocessed devices. Advances in sterilization technology and automated cleaning systems have further improved efficiency and safety, driving wider acceptance among healthcare providers.
Concerns about device safety and efficacy remain a restraint, as some providers remain hesitant to adopt reprocessed single-use devices. The complexity of sterilizing intricate devices and ensuring full traceability also adds operational challenges. Variability in regulations across European countries creates inconsistencies in adoption rates, with some nations being more restrictive than others.
Furthermore, resistance from original equipment manufacturers (OEMs), who may see reprocessing as competition, can slow adoption. Building awareness and trust among healthcare professionals is essential for overcoming these challenges.
Expansion of third-party reprocessing services across Europe presents significant opportunities. With hospitals increasingly outsourcing reprocessing to specialized providers, the market for third-party services is expected to grow rapidly. Development of advanced tracking technologies such as RFID systems provides opportunities to enhance compliance and traceability.
Opportunities also exist in expanding the range of devices eligible for reprocessing, including surgical instruments, orthopedic devices, and minimally invasive tools. As hospitals look for comprehensive reprocessing programs, vendors offering broad service portfolios will have a competitive advantage.

Market dynamics reflect a balance of cost pressures, sustainability goals, and regulatory frameworks. Adoption rates are rising, with over 45% of European hospitals already using reprocessed devices. Technological innovation in sterilization and automated reprocessing equipment is improving safety and efficiency, while increasing demand from cardiology and orthopedic procedures is driving volume growth.
Healthcare systems are under pressure to manage both financial and environmental performance, making reprocessing a valuable solution. Partnerships between hospitals and third-party reprocessors are becoming critical to meet growing demand and ensure compliance.
The research methodology for analyzing the Europe medical device reprocessing market combines both primary and secondary approaches. Primary research involves interviews with hospital administrators, reprocessing companies, regulatory experts, and clinicians. Secondary research includes government regulations, peer-reviewed publications, company filings, and industry reports.
MarkWide Research uses data triangulation and proprietary forecasting models to validate findings and provide accurate growth projections. The methodology ensures comprehensive coverage of market dynamics, adoption trends, and technological developments.
Western Europe dominates the market, with over 60% of hospitals in Germany, France, and the UK actively considering or implementing reprocessing programs. Southern Europe shows growing adoption, particularly in Italy and Spain, where hospitals seek to reduce procurement costs.
Northern Europe is experiencing strong growth at 11% CAGR, driven by sustainability mandates and advanced healthcare infrastructure. Eastern Europe remains in an early adoption stage, with rising awareness and pilot programs in countries such as Poland and Hungary expected to drive future growth.
By Type:
By Device Category:
By End User:
Cardiology devices dominate the market, accounting for 40% of total demand due to high usage in electrophysiology and catheterization procedures. Orthopedic reprocessing is gaining traction as hospitals look to reduce costs of implants and surgical instruments. General surgical instruments also represent a significant growth category, supported by rising demand for minimally invasive procedures across Europe.
Strengths:
Weaknesses:
Opportunities:
Threats:
Green healthcare initiatives are driving adoption of reprocessing to reduce carbon footprints. Third-party outsourcing is becoming the dominant model due to efficiency and compliance assurance. Technological innovation, including RFID tracking, is enhancing safety and transparency. Additionally, hospital group purchasing organizations (GPOs) are increasingly negotiating reprocessing partnerships to lower procurement costs.
The Europe medical device reprocessing market is poised for robust expansion as hospitals seek solutions that combine cost containment with environmental responsibility. Adoption will continue to rise as regulatory frameworks harmonize and confidence in safety and performance increases. Growth opportunities lie in expanding the scope of devices eligible for reprocessing, integrating digital tracking technologies, and strengthening collaboration between healthcare providers and reprocessing companies. With sustainability becoming a central healthcare goal, reprocessing will remain an essential strategy in Europe’s healthcare future.
The Europe medical device reprocessing market is entering a phase of accelerated growth, supported by cost-efficiency needs, sustainability imperatives, and regulatory backing. Hospitals across the region are increasingly adopting reprocessed devices to reduce waste and optimize budgets without compromising patient safety. While challenges such as OEM resistance and regulatory variability remain, opportunities in technology innovation and service expansion promise strong future growth. As emphasized by MarkWide Research, the market will play a central role in reshaping European healthcare practices by aligning economic efficiency with environmental sustainability, ensuring long-term value for all stakeholders.
What is Medical Device Reprocessing?
Medical Device Reprocessing refers to the process of cleaning, disinfecting, and sterilizing medical devices so they can be safely reused. This practice is essential in healthcare settings to reduce waste and ensure the availability of necessary instruments.
What are the key players in the Europe Medical Device Reprocessing market?
Key players in the Europe Medical Device Reprocessing market include Stryker Corporation, Steris plc, and Getinge AB, among others. These companies are known for their innovative solutions and technologies in the reprocessing of medical devices.
What are the main drivers of the Europe Medical Device Reprocessing market?
The main drivers of the Europe Medical Device Reprocessing market include the increasing focus on cost reduction in healthcare, the rising demand for sustainable practices, and the growing awareness of infection control. These factors contribute to the adoption of reprocessed medical devices across various healthcare facilities.
What challenges does the Europe Medical Device Reprocessing market face?
The Europe Medical Device Reprocessing market faces challenges such as stringent regulatory requirements, concerns regarding the safety and efficacy of reprocessed devices, and the need for advanced technology to ensure proper reprocessing. These challenges can hinder market growth and adoption.
What opportunities exist in the Europe Medical Device Reprocessing market?
Opportunities in the Europe Medical Device Reprocessing market include advancements in sterilization technologies, increasing partnerships between hospitals and reprocessing companies, and the potential for expanding into emerging markets. These factors can enhance the market landscape and drive growth.
What trends are shaping the Europe Medical Device Reprocessing market?
Trends shaping the Europe Medical Device Reprocessing market include the integration of automation in reprocessing facilities, the rise of eco-friendly practices, and the development of new materials that enhance the safety of reprocessed devices. These trends are influencing how healthcare providers approach device reprocessing.
Europe Medical Device Reprocessing market
| Segmentation Details | Description |
|---|---|
| Product Type | Surgical Instruments, Endoscopes, Catheters, Trays |
| Technology | Steam Sterilization, Ethylene Oxide, Hydrogen Peroxide, Ozone |
| End User | Hospitals, Clinics, Ambulatory Surgical Centers, Laboratories |
| Application | Orthopedics, Cardiology, Gastroenterology, Urology |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Europe Medical Device Reprocessing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at