444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
The Europe kidney cancer therapeutics market represents a critical segment of the continent’s oncology landscape, addressing one of the most challenging urological malignancies. Kidney cancer, primarily renal cell carcinoma, affects thousands of patients across European nations annually, driving substantial demand for innovative therapeutic solutions. The market encompasses a comprehensive range of treatment modalities including targeted therapies, immunotherapies, chemotherapy agents, and emerging precision medicine approaches.
Market dynamics in Europe reflect the region’s commitment to advanced healthcare delivery and regulatory excellence. The European Medicines Agency’s rigorous approval processes ensure that only the most effective and safe therapeutic options reach patients, while simultaneously fostering innovation among pharmaceutical manufacturers. Growth trajectories indicate robust expansion driven by increasing incidence rates, aging demographics, and breakthrough therapeutic developments.
Regional variations across European markets create diverse opportunities and challenges. Western European countries demonstrate higher adoption rates of novel therapeutics, supported by comprehensive healthcare systems and favorable reimbursement policies. Meanwhile, Eastern European markets present emerging opportunities as healthcare infrastructure modernization accelerates. The market experiences consistent growth at approximately 6.2% CAGR, reflecting both clinical needs and therapeutic advancement momentum.
The Europe kidney cancer therapeutics market refers to the comprehensive ecosystem of pharmaceutical products, treatment protocols, and healthcare services specifically designed to address kidney cancer across European territories. This market encompasses all therapeutic interventions from initial diagnosis through advanced disease management, including both curative and palliative care approaches.
Therapeutic categories within this market include targeted molecular therapies that inhibit specific cancer pathways, immunotherapy agents that harness the body’s immune system, traditional chemotherapy regimens, and supportive care medications. The market also incorporates diagnostic tools, biomarker testing, and personalized medicine approaches that guide treatment selection based on individual patient characteristics and tumor profiles.
Stakeholder involvement spans pharmaceutical manufacturers, biotechnology companies, healthcare providers, regulatory agencies, and patient advocacy organizations. Each entity contributes to the market’s evolution through research and development, clinical trials, treatment delivery, regulatory oversight, and patient support initiatives that collectively advance kidney cancer care standards across Europe.
Market leadership in Europe’s kidney cancer therapeutics sector is characterized by intense competition among established pharmaceutical giants and innovative biotechnology companies. The landscape features multiple therapeutic classes competing for market share, with immunotherapy and targeted therapy segments demonstrating particularly strong performance. Treatment paradigms continue evolving as clinical evidence supports combination therapies and personalized treatment approaches.
Regulatory environment across European markets maintains high standards for therapeutic approval while facilitating accelerated pathways for breakthrough treatments. The European Medicines Agency’s centralized approval process enables efficient market access across member states, though individual countries maintain distinct reimbursement and pricing mechanisms. Healthcare systems demonstrate varying degrees of adoption for novel therapeutics, influenced by economic considerations and clinical guidelines.
Innovation drivers include advancing understanding of kidney cancer biology, biomarker identification, and combination therapy optimization. Clinical research activities across European academic centers and pharmaceutical companies continue generating evidence supporting new treatment strategies. Patient outcomes show measurable improvements with approximately 35% of patients achieving extended progression-free survival with modern therapeutic regimens compared to historical standards.
Therapeutic advancement represents the primary catalyst driving market evolution in European kidney cancer treatment. The following insights characterize current market dynamics:
Demographic trends across Europe significantly influence kidney cancer therapeutics demand. The continent’s aging population correlates with increased cancer incidence, as kidney cancer predominantly affects individuals over 60 years of age. Lifestyle factors including smoking, obesity, and hypertension contribute to rising disease prevalence, creating sustained demand for therapeutic interventions.
Scientific breakthroughs in cancer biology and immunology drive therapeutic innovation. Understanding of tumor microenvironments, immune system interactions, and molecular pathways enables development of more targeted and effective treatments. Research investments from both public and private sectors support continued advancement in therapeutic options, with European pharmaceutical companies leading global innovation efforts.
Healthcare infrastructure improvements across European markets facilitate better disease detection and treatment delivery. Enhanced diagnostic capabilities enable earlier cancer identification, while specialized oncology centers provide comprehensive care coordination. Regulatory support through expedited approval pathways for breakthrough therapies accelerates patient access to innovative treatments, with approximately 42% of new approvals receiving accelerated review status.
Economic factors including healthcare spending increases and favorable reimbursement policies support market growth. European governments recognize the long-term economic benefits of effective cancer treatment, leading to improved coverage for novel therapeutics. Patient advocacy efforts also contribute to market expansion by raising awareness and supporting research funding initiatives.
Cost considerations present significant challenges for kidney cancer therapeutics market expansion across Europe. Novel immunotherapies and targeted agents command premium pricing, creating budget pressures for healthcare systems already managing resource constraints. Reimbursement delays and complex health technology assessment processes can limit patient access to innovative treatments, particularly in cost-sensitive markets.
Regulatory complexity across different European jurisdictions creates market access challenges for pharmaceutical companies. While the European Medicines Agency provides centralized approval, individual countries maintain distinct pricing and reimbursement mechanisms. Administrative burdens associated with multiple regulatory requirements can delay market entry and increase operational costs for therapeutic developers.
Treatment resistance remains a clinical challenge that limits therapeutic effectiveness. Many patients eventually develop resistance to targeted therapies and immunotherapies, necessitating treatment switches and combination approaches. Side effect profiles of intensive treatment regimens can limit patient tolerance and treatment continuation, affecting overall therapeutic success rates.
Healthcare disparities across European regions create uneven market development opportunities. Rural areas and economically disadvantaged regions may have limited access to specialized oncology care and novel therapeutics. Physician expertise variations in kidney cancer management can influence treatment adoption rates, with approximately 28% of patients receiving suboptimal initial therapy selection due to limited specialist availability.
Emerging markets within Eastern Europe present substantial growth opportunities as healthcare systems modernize and cancer care capabilities expand. Countries investing in oncology infrastructure create new demand for advanced therapeutics. Market penetration strategies targeting these developing markets can yield significant returns as economic conditions improve and healthcare access increases.
Combination therapy development represents a major opportunity for pharmaceutical companies to differentiate their products and improve patient outcomes. Clinical research exploring novel drug combinations continues revealing synergistic effects that enhance therapeutic efficacy. Companies successfully developing effective combination regimens can capture premium market positions and extended patent protection periods.
Precision medicine advancement creates opportunities for companion diagnostics and personalized treatment approaches. Biomarker identification enables more targeted therapeutic selection, improving success rates while reducing unnecessary treatment exposure. The integration of genetic testing and molecular profiling into standard care protocols expands market opportunities for both therapeutics and diagnostic companies.
Digital health integration offers opportunities to enhance treatment monitoring and patient management. Telemedicine platforms and remote monitoring technologies can improve treatment adherence and early detection of adverse events. Companies developing integrated digital health solutions alongside therapeutic products can create competitive advantages and improve patient outcomes, with 67% of oncologists expressing interest in digital treatment support tools.
Competitive intensity within the European kidney cancer therapeutics market drives continuous innovation and strategic positioning among pharmaceutical companies. Market leaders maintain positions through robust clinical evidence, comprehensive market access strategies, and strong relationships with key opinion leaders. New entrants challenge established players by developing novel mechanisms of action and targeting unmet clinical needs.
Treatment paradigm shifts influence market dynamics as clinical evidence supports new therapeutic approaches. The transition from traditional chemotherapy to immunotherapy and targeted agents represents a fundamental change in kidney cancer management. Physician adoption of new treatment protocols varies across European markets, influenced by clinical guidelines, reimbursement policies, and local medical practices.
Supply chain considerations affect market stability and product availability. Complex manufacturing requirements for biologics and specialized storage needs for certain therapeutics create logistical challenges. Distribution networks must accommodate cold chain requirements and ensure reliable product delivery to healthcare facilities across diverse European markets.
Patient advocacy influence shapes market dynamics through awareness campaigns, research funding support, and policy advocacy efforts. Clinical outcomes data increasingly drives treatment decisions, with real-world evidence supplementing clinical trial results. Market dynamics also reflect the growing emphasis on patient-reported outcomes and quality of life measures in therapeutic evaluation, with 73% of treatment decisions incorporating patient preference considerations.
Comprehensive analysis of the European kidney cancer therapeutics market employs multiple research methodologies to ensure accuracy and reliability. Primary research includes extensive interviews with key stakeholders including oncologists, pharmaceutical executives, regulatory officials, and patient advocacy representatives across major European markets. These interviews provide insights into market trends, treatment patterns, and future opportunities.
Secondary research encompasses analysis of clinical trial databases, regulatory filings, medical literature, and industry reports. Data validation processes ensure information accuracy through cross-referencing multiple sources and expert verification. Market sizing and forecasting utilize established econometric models adapted for pharmaceutical market analysis, incorporating factors such as patient populations, treatment rates, and pricing dynamics.
Regional analysis methodology includes country-specific assessments of healthcare systems, regulatory environments, and market access conditions. Competitive intelligence gathering involves monitoring pharmaceutical company activities, pipeline developments, and strategic initiatives. Market research also incorporates analysis of reimbursement policies, health technology assessments, and healthcare spending patterns across European jurisdictions.
MarkWide Research employs proprietary analytical frameworks to assess market dynamics and competitive positioning. Forecasting models integrate multiple variables including demographic trends, clinical development timelines, and regulatory approval probabilities to generate reliable market projections and identify emerging opportunities within the therapeutic landscape.
Western European markets dominate the kidney cancer therapeutics landscape, led by Germany, France, and the United Kingdom. These markets demonstrate high adoption rates for novel therapeutics, supported by robust healthcare systems and favorable reimbursement environments. Germany represents the largest single market, accounting for approximately 23% of European therapeutic consumption, driven by comprehensive cancer care infrastructure and strong pharmaceutical industry presence.
Nordic countries including Sweden, Norway, and Denmark exhibit exceptional treatment outcomes and high per-capita therapeutic utilization. These markets prioritize innovative cancer treatments and maintain efficient healthcare delivery systems. Reimbursement policies in Nordic countries typically support rapid adoption of proven therapeutic advances, creating attractive market conditions for pharmaceutical companies.
Southern European markets including Italy and Spain present mixed opportunities, with strong clinical expertise but varying economic conditions affecting market access. Healthcare budget constraints in some regions create challenges for premium-priced therapeutics, though clinical need drives continued market growth. These markets increasingly adopt value-based pricing models that align therapeutic costs with patient outcomes.
Eastern European markets demonstrate rapid growth potential as healthcare systems modernize and cancer care capabilities expand. Countries such as Poland and Czech Republic invest significantly in oncology infrastructure, creating new demand for advanced therapeutics. Market penetration in Eastern Europe currently represents approximately 18% of total European consumption, with substantial growth opportunities as economic development continues.
Market leadership in European kidney cancer therapeutics involves several major pharmaceutical companies competing across different therapeutic segments. The competitive environment features both established oncology specialists and emerging biotechnology companies developing innovative treatment approaches.
Strategic positioning among competitors involves differentiation through clinical efficacy, safety profiles, and market access capabilities. Innovation focus centers on combination therapies, biomarker-driven treatments, and next-generation therapeutic mechanisms that address current treatment limitations.
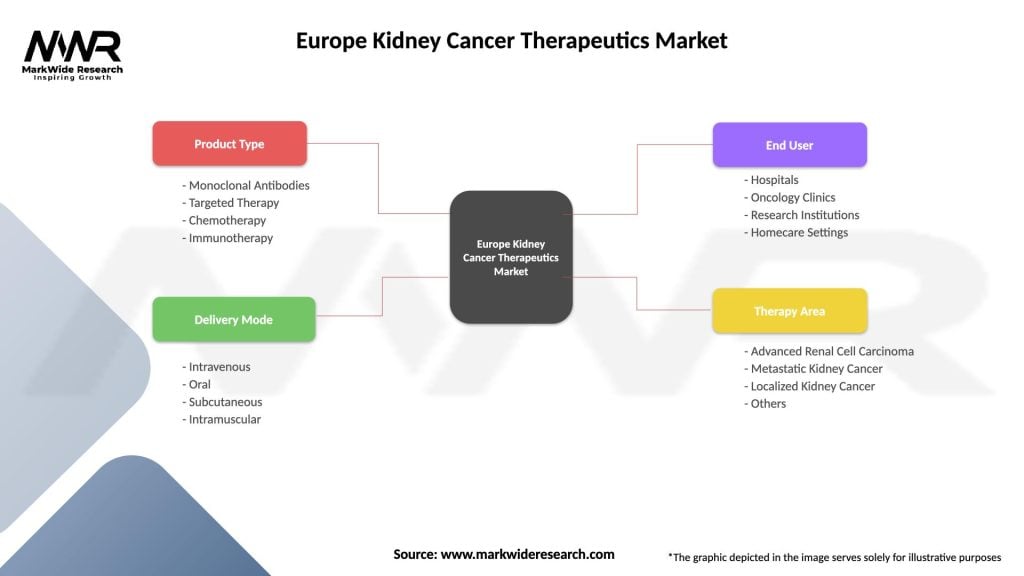
Therapeutic class segmentation divides the European kidney cancer therapeutics market into distinct categories based on mechanism of action and clinical application. Each segment demonstrates unique growth characteristics and competitive dynamics.
By Drug Class:
By Cancer Stage:
By Distribution Channel:
Immunotherapy segment demonstrates exceptional growth momentum, driven by checkpoint inhibitors that revolutionize kidney cancer treatment outcomes. Clinical evidence supporting immunotherapy efficacy continues expanding, with combination approaches showing particular promise. This segment captures approximately 45% market share across European markets, reflecting strong physician adoption and patient outcomes.
Targeted therapy category maintains significant market presence through established efficacy in specific patient populations. Precision medicine approaches within this segment enable personalized treatment selection based on molecular characteristics. Ongoing research focuses on overcoming resistance mechanisms and identifying optimal patient selection criteria for improved therapeutic success rates.
Combination therapy approaches represent the future direction of kidney cancer treatment, combining different mechanisms of action to enhance efficacy while managing toxicity profiles. Clinical development in this category focuses on identifying optimal drug combinations, sequencing strategies, and patient selection criteria. Regulatory agencies increasingly support combination therapy development through adaptive trial designs and expedited approval pathways.
Supportive care segment provides essential services including symptom management, nutritional support, and quality of life enhancement. Integrated care approaches recognize the importance of comprehensive patient support throughout treatment journeys. This segment demonstrates steady growth as treatment protocols become more intensive and patient survival extends, requiring longer-term supportive interventions.
Pharmaceutical companies benefit from substantial revenue opportunities within the European kidney cancer therapeutics market. Innovation rewards include premium pricing for breakthrough therapies and extended market exclusivity periods. Companies successfully developing effective treatments gain competitive advantages through strong clinical evidence and physician relationships.
Healthcare providers benefit from improved patient outcomes and enhanced treatment options that enable personalized care approaches. Clinical benefits include extended patient survival, improved quality of life, and reduced disease progression rates. Healthcare systems also benefit from potential long-term cost savings through more effective treatments that reduce hospitalization needs and disease complications.
Patients and families experience the most significant benefits through improved survival outcomes and enhanced quality of life during treatment. Treatment advances provide hope for previously difficult-to-treat conditions while offering less toxic alternatives to traditional chemotherapy. Patient support programs associated with novel therapeutics provide additional resources for managing treatment challenges.
Research institutions benefit from collaboration opportunities with pharmaceutical companies and access to cutting-edge therapeutic development programs. Academic benefits include research funding, publication opportunities, and contributions to medical knowledge advancement. Clinical research activities also enhance institutional reputations and attract top medical talent, with 56% of leading cancer centers reporting increased research collaboration opportunities.
Strengths:
Weaknesses:
Opportunities:
Threats:
Immunotherapy evolution continues driving market transformation as new checkpoint inhibitors and immune system modulators enter clinical development. Treatment protocols increasingly incorporate immunotherapy as first-line treatment, fundamentally changing kidney cancer management approaches. Clinical research focuses on optimizing immunotherapy combinations and identifying predictive biomarkers for treatment response.
Precision medicine integration represents a major trend reshaping therapeutic selection and patient management strategies. Molecular profiling becomes standard practice for treatment planning, enabling personalized approaches that improve efficacy while minimizing unnecessary treatment exposure. Companion diagnostics development accelerates to support precision medicine implementation across European healthcare systems.
Digital health adoption transforms patient monitoring and treatment support services throughout the therapeutic journey. Remote monitoring technologies enable early detection of adverse events and treatment response assessment. Telemedicine platforms facilitate specialist consultation access in underserved regions, improving treatment standardization across European markets.
Value-based care models increasingly influence therapeutic adoption and reimbursement decisions across European healthcare systems. Outcomes-based pricing aligns therapeutic costs with patient benefits, creating incentives for pharmaceutical companies to demonstrate real-world effectiveness. This trend drives development of comprehensive patient support programs and outcomes measurement systems, with 38% of new therapeutic approvals incorporating value-based pricing elements.
Regulatory approvals for novel therapeutic combinations continue expanding treatment options for European kidney cancer patients. Recent approvals include immunotherapy combinations that demonstrate superior efficacy compared to single-agent treatments. European Medicines Agency expedited review processes facilitate faster patient access to breakthrough therapies while maintaining rigorous safety standards.
Clinical trial innovations include adaptive trial designs that accelerate therapeutic development and improve patient access to experimental treatments. Biomarker-driven trials enable more efficient patient selection and faster identification of effective treatment approaches. European clinical research networks facilitate multinational studies that generate robust evidence for regulatory approval and clinical practice guidelines.
Strategic partnerships between pharmaceutical companies, biotechnology firms, and academic institutions accelerate therapeutic development and market access initiatives. Collaboration models include risk-sharing agreements, co-development programs, and licensing arrangements that optimize resource utilization and expertise sharing. These partnerships particularly focus on combination therapy development and precision medicine approaches.
Manufacturing investments across European markets ensure reliable supply of complex biologics and specialized therapeutics. Production capacity expansion supports growing demand while maintaining quality standards required for oncology therapeutics. Supply chain resilience initiatives address potential disruptions and ensure consistent product availability across diverse European markets, with MarkWide Research indicating significant infrastructure investment growth in the region.
Market entry strategies for pharmaceutical companies should prioritize countries with favorable reimbursement environments and established oncology infrastructure. Germany and France represent optimal initial markets due to their size, healthcare sophistication, and supportive regulatory environments. Companies should develop comprehensive market access strategies that address both clinical evidence requirements and health economic considerations.
Product development focus should emphasize combination therapies and precision medicine approaches that address current treatment limitations. Biomarker development alongside therapeutic agents creates competitive advantages and supports premium pricing strategies. Companies should invest in companion diagnostics and patient selection strategies that optimize therapeutic success rates.
Partnership opportunities with European academic institutions and healthcare systems can accelerate clinical development and market acceptance. Collaborative research generates real-world evidence that supports regulatory approval and clinical guideline development. Strategic alliances with established European pharmaceutical companies can provide market access expertise and distribution capabilities.
Digital health integration should be incorporated into therapeutic development and commercialization strategies to enhance patient outcomes and differentiate products. Remote monitoring capabilities and patient support platforms create additional value propositions beyond therapeutic efficacy. Companies should consider partnerships with digital health providers to develop comprehensive treatment solutions that address evolving healthcare delivery models.
Market evolution over the next decade will be characterized by continued therapeutic innovation and expanding treatment applications. Pipeline developments include next-generation immunotherapies, novel targeted agents, and innovative combination approaches that promise further improvements in patient outcomes. Clinical research activities across European institutions continue generating evidence supporting new treatment paradigms.
Precision medicine advancement will transform kidney cancer treatment through increasingly sophisticated patient selection and treatment optimization strategies. Biomarker identification and validation efforts will enable more targeted therapeutic approaches that maximize efficacy while minimizing adverse events. Integration of artificial intelligence and machine learning technologies will enhance treatment decision-making and outcome prediction capabilities.
Market access evolution will reflect growing emphasis on value-based healthcare and outcomes measurement. Reimbursement policies will increasingly incorporate real-world evidence and patient-reported outcomes in coverage decisions. Health technology assessment processes will evolve to accommodate innovative therapeutic approaches and combination regimens that demonstrate superior clinical value.
Geographic expansion opportunities will emerge as Eastern European markets continue healthcare system modernization and cancer care capability development. MWR analysis projects sustained market growth at approximately 7.1% CAGR over the forecast period, driven by demographic trends, therapeutic innovation, and expanding market access across European territories. The market will continue evolving toward personalized, combination-based treatment approaches that optimize individual patient outcomes while managing healthcare system sustainability.
The European kidney cancer therapeutics market represents a dynamic and rapidly evolving landscape characterized by significant therapeutic innovation and expanding treatment opportunities. Market growth is driven by demographic trends, scientific advancement, and healthcare system support for innovative cancer treatments. The transition from traditional chemotherapy to immunotherapy and targeted agents fundamentally transforms patient outcomes and market dynamics.
Competitive positioning within this market requires comprehensive strategies that address clinical efficacy, regulatory approval, market access, and patient support considerations. Successful companies demonstrate strong clinical evidence, effective market access capabilities, and commitment to addressing unmet medical needs. The market rewards innovation while demanding rigorous safety and efficacy standards that protect patient welfare.
Future opportunities center on precision medicine advancement, combination therapy development, and geographic expansion into emerging European markets. Digital health integration and value-based care models will increasingly influence market dynamics and competitive positioning. Companies that successfully navigate these trends while maintaining focus on patient outcomes will capture the greatest opportunities within this essential healthcare market segment.
What is Kidney Cancer Therapeutics?
Kidney Cancer Therapeutics refers to the various treatments and medications used to manage and treat kidney cancer, including targeted therapies, immunotherapies, and chemotherapy options.
What are the key players in the Europe Kidney Cancer Therapeutics Market?
Key players in the Europe Kidney Cancer Therapeutics Market include Bristol-Myers Squibb, Merck & Co., Novartis, and Pfizer, among others.
What are the main drivers of growth in the Europe Kidney Cancer Therapeutics Market?
The growth of the Europe Kidney Cancer Therapeutics Market is driven by increasing incidence rates of kidney cancer, advancements in treatment technologies, and a growing focus on personalized medicine.
What challenges does the Europe Kidney Cancer Therapeutics Market face?
Challenges in the Europe Kidney Cancer Therapeutics Market include high treatment costs, regulatory hurdles, and the need for ongoing research to address drug resistance in patients.
What opportunities exist in the Europe Kidney Cancer Therapeutics Market?
Opportunities in the Europe Kidney Cancer Therapeutics Market include the development of novel therapies, expansion into emerging markets, and increasing collaborations between pharmaceutical companies and research institutions.
What trends are shaping the Europe Kidney Cancer Therapeutics Market?
Trends in the Europe Kidney Cancer Therapeutics Market include the rise of combination therapies, the use of biomarker testing for treatment selection, and a shift towards more patient-centric care approaches.
Europe Kidney Cancer Therapeutics Market
| Segmentation Details | Description |
|---|---|
| Product Type | Monoclonal Antibodies, Targeted Therapy, Chemotherapy, Immunotherapy |
| Delivery Mode | Intravenous, Oral, Subcutaneous, Intramuscular |
| End User | Hospitals, Oncology Clinics, Research Institutions, Homecare Settings |
| Therapy Area | Advanced Renal Cell Carcinoma, Metastatic Kidney Cancer, Localized Kidney Cancer, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Europe Kidney Cancer Therapeutics Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at