444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Europe generic injectables market refers to the pharmaceutical sector that focuses on the production and distribution of generic injectable medications across European countries. Generic injectables are drugs that are bioequivalent to their brand-name counterparts, but they are usually available at a lower cost. These medications are administered through injections, providing an effective and rapid route for drug delivery.
Meaning
Generic injectables are a crucial part of the healthcare system in Europe. They offer cost-effective alternatives to branded injectable medications, making healthcare more accessible and affordable for patients. These medications play a vital role in various medical settings, including hospitals, clinics, and home healthcare.
Executive Summary
The Europe generic injectables market has experienced significant growth in recent years. The rising demand for affordable healthcare solutions, coupled with the increasing prevalence of chronic diseases, has fueled the market’s expansion. Moreover, the expiration of patents for several branded injectables has opened doors for generic manufacturers, boosting competition and driving market growth.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
The Europe generic injectables market is driven by various factors, including:
Market Restraints
Despite its growth potential, the Europe generic injectables market faces certain challenges, including:
Market Opportunities
The Europe generic injectables market presents several opportunities for growth, such as:
Market Dynamics
The Europe generic injectables market is dynamic and influenced by various factors, including changing patient demographics, evolving regulatory landscapes, and advancements in pharmaceutical technologies. Market players need to adapt to these dynamics to stay competitive and meet the evolving needs of healthcare providers and patients.
Regional Analysis
The Europe generic injectables market can be segmented into various regions, including Western Europe and Eastern Europe. Western European countries, such as Germany, France, and the United Kingdom, have well-established healthcare systems and account for a significant market share. On the other hand, Eastern European countries, including Poland, Romania, and Hungary, offer growth opportunities due to increasing healthcare investments and the rising demand for affordable medications.
Competitive Landscape
Leading Companies in the Europe Generic Injectables Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
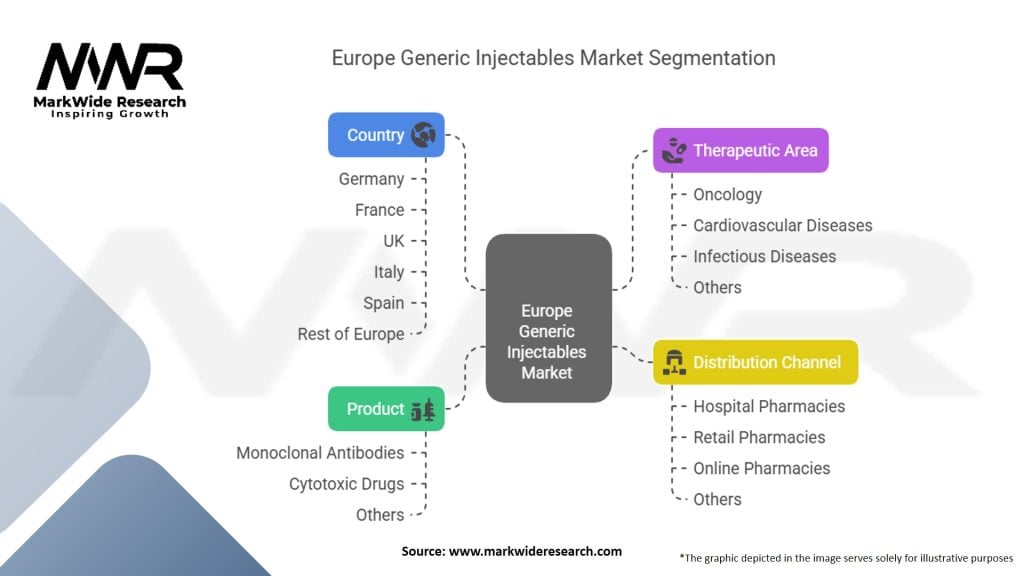
Segmentation
The Europe generic injectables market can be segmented based on product type, therapeutic area, distribution channel, and geography. Product types may include small molecules and biosimilars, while therapeutic areas encompass oncology, cardiovascular diseases, infectious diseases, and others. Distribution channels include hospitals, clinics, and retail pharmacies.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the Europe generic injectables market can benefit in the following ways:
SWOT Analysis
The Europe generic injectables market can be analyzed using a SWOT (Strengths, Weaknesses, Opportunities, and Threats) framework.
Market Key Trends
The Europe generic injectables market is witnessing several key trends:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Europe generic injectables market. The increased demand for injectable medications, such as antivirals and immunosuppressants, to manage COVID-19 infections has led to a surge in production and consumption. However, disruptions in the supply chain, lockdown measures, and strained healthcare systems have posed challenges for market players. Despite these obstacles, the pandemic has highlighted the importance of affordable and accessible generic injectables in managing public health emergencies.
Key Industry Developments
The Europe generic injectables market has witnessed several key industry developments:
Analyst Suggestions
Based on market trends and dynamics, analysts suggest the following strategies for industry participants:
Future Outlook
The Europe generic injectables market is expected to continue its growth trajectory in the coming years. Factors such as increasing healthcare costs, patent expirations, and the need for affordable medications will drive market expansion. Technological advancements, collaborations, and the focus on biosimilars will further shape the market landscape. However, industry participants must navigate challenges related to regulatory compliance, competition, and market access to capitalize on future opportunities.
Conclusion
The Europe generic injectables market is witnessing significant growth due to the increasing demand for cost-effective medications, patent expirations, and the expanding geriatric population. While facing challenges such as stringent regulations and competition from branded medications, the market offers ample opportunities for innovation, partnerships, and market expansion. With the ongoing impact of COVID-19 and the evolving healthcare landscape, industry players need to adapt to changing dynamics and focus on delivering high-quality, affordable generic injectables to meet the growing healthcare needs of the European population.
What are Europe Generic Injectables?
Europe Generic Injectables refer to pharmaceutical products that are chemically identical to their branded counterparts but are sold under their chemical names or as generic brands. These injectables are used in various therapeutic areas, including oncology, anesthesia, and infectious diseases.
Who are the key players in the Europe Generic Injectables Market?
Key players in the Europe Generic Injectables Market include companies like Sandoz, Teva Pharmaceutical Industries, and Fresenius Kabi, among others. These companies are known for their extensive portfolios of generic injectable products and their commitment to quality and affordability.
What are the main drivers of growth in the Europe Generic Injectables Market?
The growth of the Europe Generic Injectables Market is driven by factors such as the increasing prevalence of chronic diseases, the rising demand for cost-effective treatment options, and the expiration of patents for several branded injectables. Additionally, the focus on improving healthcare access contributes to market expansion.
What challenges does the Europe Generic Injectables Market face?
The Europe Generic Injectables Market faces challenges such as stringent regulatory requirements, pricing pressures from healthcare systems, and the complexity of manufacturing processes. These factors can hinder the entry of new players and affect the profitability of existing companies.
What opportunities exist in the Europe Generic Injectables Market?
Opportunities in the Europe Generic Injectables Market include the potential for biosimilars, advancements in drug delivery technologies, and the growing trend of personalized medicine. These factors can lead to the development of innovative injectable products that meet specific patient needs.
What trends are shaping the Europe Generic Injectables Market?
Trends shaping the Europe Generic Injectables Market include the increasing adoption of automated manufacturing processes, the rise of telemedicine, and the growing emphasis on sustainability in pharmaceutical production. These trends are influencing how generic injectables are developed and distributed.
Europe Generic Injectables Market:
| Segmentation Details | Description |
|---|---|
| Product | Monoclonal Antibodies, Cytotoxic Drugs, Others |
| Therapeutic Area | Oncology, Cardiovascular Diseases, Infectious Diseases, Others |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Others |
| Country | Germany, France, UK, Italy, Spain, Rest of Europe |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Europe Generic Injectables Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at