444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview:
The Europe External Defibrillators Market is a crucial segment within the broader medical devices industry, playing a pivotal role in cardiac emergency response. External defibrillators are life-saving devices designed to deliver controlled electric shocks to the heart, restoring normal cardiac rhythm in cases of sudden cardiac arrest. The market’s growth is driven by the increasing incidence of cardiovascular diseases, advancements in defibrillator technology, and the rising awareness about the importance of early defibrillation in emergency medical care.
Meaning:
External defibrillators are portable electronic devices used to diagnose and treat life-threatening cardiac arrhythmias, particularly ventricular fibrillation and ventricular tachycardia. These devices analyze the heart’s electrical activity and deliver therapeutic shocks to restore a normal rhythm. External defibrillators are widely used in various settings, including hospitals, clinics, public spaces, and homes, to provide timely intervention during sudden cardiac emergencies.
Executive Summary:
The Europe External Defibrillators Market has witnessed significant growth in recent years, driven by factors such as increasing cardiovascular disease prevalence, advancements in defibrillator technology, and the implementation of public access defibrillation programs. The market is characterized by the presence of established medical device manufacturers, technological innovation, and a focus on enhancing user-friendly features in defibrillator devices. As countries in Europe prioritize public health and emergency response systems, the demand for external defibrillators is expected to continue growing.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Europe External Defibrillators Market operates in a dynamic environment shaped by factors such as technological advancements, regulatory developments, public health initiatives, and market competition. Understanding these dynamics is crucial for stakeholders to navigate challenges and capitalize on opportunities.
Regional Analysis:
The Europe External Defibrillators Market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory frameworks, and the prevalence of cardiovascular diseases. Key regions include:
Competitive Landscape:
Leading Companies in Europe External Defibrillators Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
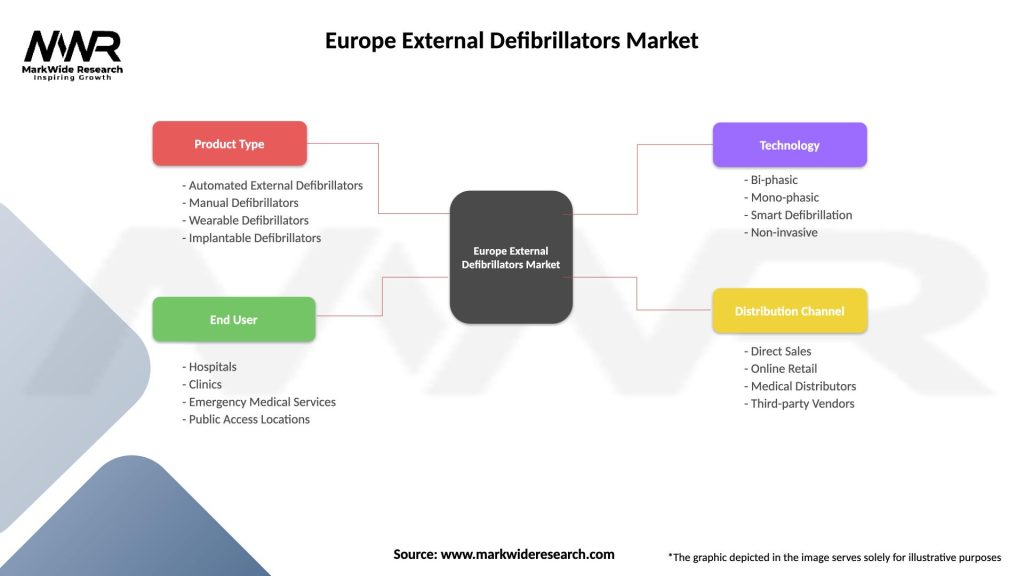
Segmentation: The Europe External Defibrillators Market can be segmented based on various factors, including:
Segmentation provides a comprehensive understanding of market dynamics and allows stakeholders to tailor strategies based on specific market segments.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders: The Europe External Defibrillators Market offers several benefits for industry participants and stakeholders:
SWOT Analysis: A SWOT analysis provides insights into the Europe External Defibrillators Market’s strengths, weaknesses, opportunities, and threats:
Understanding these factors through a SWOT analysis helps industry participants navigate challenges, leverage strengths, and capitalize on opportunities.
Market Key Trends:
Covid-19 Impact: The Covid-19 pandemic has had implications for the Europe External Defibrillators Market:
Key Industry Developments:
Analyst Suggestions:
Future Outlook
The Europe External Defibrillators Market is poised for continued growth in the coming years. Factors such as increasing awareness of cardiac health, advancements in defibrillator technology, and collaborative efforts for public access defibrillation programs will shape the market’s future. The industry’s ability to address challenges, embrace innovation, and contribute to overall healthcare preparedness will be crucial for sustained success.
Conclusion:
The Europe External Defibrillators Market presents significant opportunities and challenges, driven by factors such as the increasing prevalence of cardiovascular diseases, technological advancements, and public health initiatives. Industry participants must navigate regulatory complexities, address training challenges, and focus on innovation to meet evolving market demands. The expansion of public access defibrillation programs and the development of wearable defibrillator technologies represent key avenues for growth. Overall, the market’s dynamics require a holistic approach that combines technological innovation, strategic collaborations, and a commitment to improving emergency response systems.
What is External Defibrillators?
External defibrillators are medical devices used to restore a normal heart rhythm in individuals experiencing sudden cardiac arrest. They deliver an electric shock to the heart to re-establish effective heartbeats.
What are the key players in the Europe External Defibrillators Market?
Key players in the Europe External Defibrillators Market include Philips Healthcare, Zoll Medical Corporation, and Medtronic, among others. These companies are known for their innovative products and contributions to improving cardiac care.
What are the growth factors driving the Europe External Defibrillators Market?
The growth of the Europe External Defibrillators Market is driven by increasing awareness of sudden cardiac arrest, advancements in technology, and the rising prevalence of cardiovascular diseases. Additionally, government initiatives to promote public access to defibrillators contribute to market expansion.
What challenges does the Europe External Defibrillators Market face?
The Europe External Defibrillators Market faces challenges such as high costs of advanced devices and the need for regular maintenance and training for effective use. Additionally, regulatory hurdles can impede the introduction of new technologies.
What opportunities exist in the Europe External Defibrillators Market?
Opportunities in the Europe External Defibrillators Market include the development of portable and user-friendly devices, increasing adoption in public spaces, and the integration of smart technology for real-time monitoring. These trends can enhance accessibility and effectiveness in emergency situations.
What trends are shaping the Europe External Defibrillators Market?
Trends shaping the Europe External Defibrillators Market include the rise of automated external defibrillators (AEDs) in public areas, advancements in connectivity features, and a focus on training programs for laypersons. These trends aim to improve response times and survival rates in cardiac emergencies.
Europe External Defibrillators Market
| Segmentation Details | Description |
|---|---|
| Product Type | Automated External Defibrillators, Manual Defibrillators, Wearable Defibrillators, Implantable Defibrillators |
| End User | Hospitals, Clinics, Emergency Medical Services, Public Access Locations |
| Technology | Bi-phasic, Mono-phasic, Smart Defibrillation, Non-invasive |
| Distribution Channel | Direct Sales, Online Retail, Medical Distributors, Third-party Vendors |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at