444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Engineered T Cells market is a rapidly growing sector within the biotechnology industry. Engineered T cells are a type of immunotherapy that involves modifying a patient’s own T cells to recognize and attack cancer cells more effectively. This innovative approach has shown promising results in the treatment of various types of cancer, including leukemia, lymphoma, and solid tumors.
Meaning
Engineered T cells are genetically modified immune cells that are designed to target and destroy cancer cells in a patient’s body. The process involves extracting T cells from the patient, modifying them in the laboratory to express a specific receptor that recognizes cancer cells, and then reinfusing the modified cells back into the patient. Once inside the patient’s body, these engineered T cells can recognize and attack cancer cells, providing a personalized and targeted treatment option.
Executive Summary
The Engineered T Cells market is witnessing significant growth due to the increasing prevalence of cancer and the rising demand for more effective treatment options. The market is characterized by intense competition among key players, technological advancements in cell engineering, and growing investments in research and development. The market is expected to continue its upward trajectory in the coming years as more clinical trials demonstrate the efficacy of engineered T cells in treating cancer.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
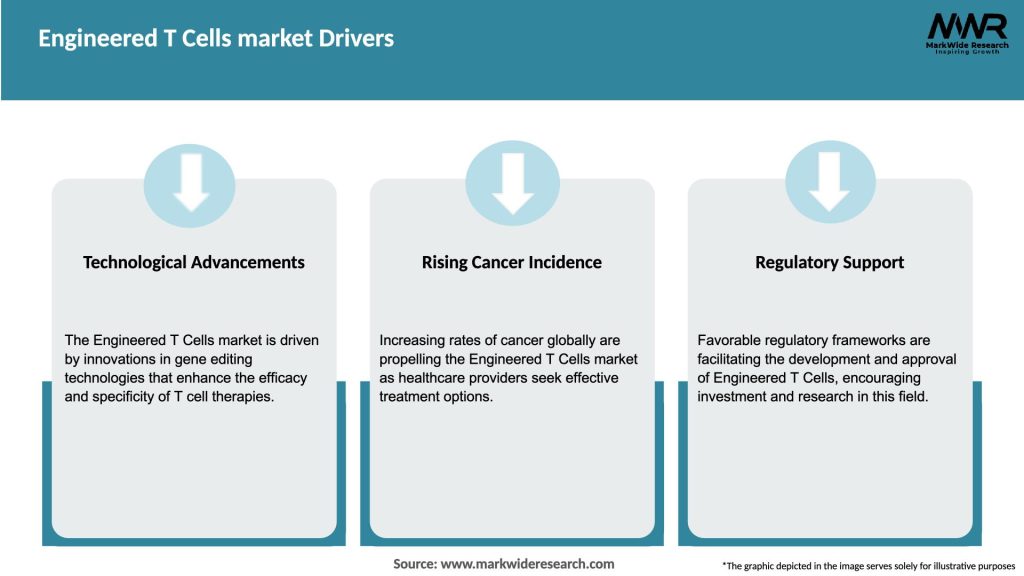
Market Drivers
The Engineered T Cells market is driven by several factors that contribute to its rapid growth. One of the primary drivers is the increasing incidence of cancer worldwide. According to the World Health Organization (WHO), cancer is one of the leading causes of death globally, with millions of new cases diagnosed each year. Engineered T cell therapies offer a promising approach to combat this deadly disease by harnessing the power of the immune system to target and eliminate cancer cells.
Another key driver is the growing demand for more effective and personalized cancer treatments. Traditional cancer treatments such as chemotherapy and radiation therapy can have significant side effects and may not always be effective, especially in advanced stages of the disease. Engineered T cell therapies have shown remarkable success in treating certain types of cancer, including hematological malignancies, providing hope for patients who have exhausted other treatment options.
Furthermore, advancements in cell engineering technologies have played a crucial role in driving market growth. The development of chimeric antigen receptor (CAR) T cell therapies has revolutionized cancer treatment. CAR-T cells are engineered to express receptors that specifically target cancer cells, enhancing their ability to recognize and destroy malignant cells. These technological advancements have significantly improved the efficacy and safety of engineered T cell therapies, driving their adoption in clinical practice.
Market Restraints
Despite the significant growth potential, the Engineered T Cells market faces certain challenges and restraints. One of the primary concerns is the high cost associated with these therapies. Engineered T cell treatments are complex and require sophisticated manufacturing processes, which contribute to their high price. The cost of manufacturing, patient-specific customization, and the need for specialized facilities all contribute to the overall expense. The high cost can limit access to these therapies, particularly in resource-limited settings, and pose a financial burden on patients and healthcare systems.
Additionally, the potential for adverse side effects is a significant concern in the field of engineered T cell therapies. While these therapies have shown remarkable efficacy, they can also cause severeside effects, including cytokine release syndrome (CRS) and neurotoxicity. CRS is a systemic inflammatory response that can lead to fever, hypotension, and organ dysfunction. Neurotoxicity, on the other hand, can manifest as confusion, seizures, and even coma. The management of these side effects requires specialized care and monitoring, adding to the complexity and cost of treatment.
Moreover, regulatory challenges and reimbursement policies can also impede market growth. The approval process for engineered T cell therapies involves stringent regulatory scrutiny due to the novel nature of these treatments. The regulatory agencies require substantial clinical data on safety and efficacy before granting market authorization. Navigating these regulatory pathways can be time-consuming and costly, hindering the timely availability of these therapies to patients.
Market Opportunities
Despite the challenges, the Engineered T Cells market presents several opportunities for growth and advancement. One such opportunity lies in expanding the application of engineered T cells to target a broader range of cancer types. While these therapies have demonstrated significant success in hematological malignancies, their efficacy in solid tumors is still being explored. Ongoing research and clinical trials are investigating the potential of engineered T cells in treating various solid tumor types, including lung, breast, and pancreatic cancer. Successful outcomes in these studies would open up new avenues for market expansion.
Another opportunity lies in the development of off-the-shelf engineered T cell therapies. Currently, most engineered T cell therapies are patient-specific and require a complex and time-consuming manufacturing process. The development of off-the-shelf products would eliminate the need for patient-specific customization, reducing manufacturing costs and turnaround time. This approach could significantly improve accessibility to these therapies and broaden their adoption in clinical practice.
Additionally, the integration of engineered T cells with other treatment modalities, such as immune checkpoint inhibitors, holds promise for improving patient outcomes. Combining different immunotherapies can enhance the immune response against cancer cells and overcome resistance mechanisms. Ongoing research in combination therapies could lead to synergistic effects and improved treatment outcomes, presenting a lucrative opportunity for market players.

Market Dynamics
The Engineered T Cells market is characterized by dynamic factors that influence its growth and evolution. Key dynamics include technological advancements, strategic collaborations, regulatory landscape, and patient awareness and acceptance.
Technological advancements in cell engineering techniques continue to drive innovation in the field of engineered T cell therapies. Researchers and industry players are constantly exploring new methods to improve the efficiency and safety of these therapies. The development of next-generation CAR-T cells, such as armored CAR-T cells and dual-targeted CAR-T cells, is a testament to the dynamic nature of the market. These advancements are aimed at overcoming existing limitations and expanding the applicability of engineered T cells in different cancer types.
Strategic collaborations and partnerships among biopharmaceutical companies, research institutions, and academic centers are another significant dynamic in the market. These collaborations enable the pooling of resources, expertise, and intellectual property to accelerate the development and commercialization of engineered T cell therapies. Such partnerships also facilitate knowledge sharing and foster innovation, strengthening the overall market landscape.
The regulatory landscape plays a crucial role in shaping the market dynamics. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play a vital role in approving and monitoring the safety and efficacy of engineered T cell therapies. The evolving regulatory environment and the increasing understanding of the risks and benefits associated with these therapies impact market dynamics. The speed and efficiency of the regulatory approval process can influence the market’s growth potential and the timelines for market entry.
Patient awareness and acceptance of engineered T cell therapies are also significant dynamics. As patients become more informed about these innovative treatment options, the demand for engineered T cell therapies is expected to increase. The patient-centric approach of personalized medicine aligns with the aspirations of patientsseeking targeted and effective cancer treatments. However, patient acceptance also depends on factors such as safety, accessibility, and affordability. Educating patients and healthcare professionals about the benefits and risks of engineered T cell therapies is essential for fostering acceptance and driving market growth.
Regional Analysis
The Engineered T Cells market exhibits a global presence, with significant regional variations in market dynamics. North America currently dominates the market, owing to factors such as advanced healthcare infrastructure, favorable reimbursement policies, and the presence of key market players. The United States, in particular, accounts for a substantial share of the global market due to its robust research and development activities and early adoption of innovative therapies.
Europe is another significant market for engineered T cells, driven by a supportive regulatory environment and increasing investments in cell-based therapies. The region has witnessed the approval of several CAR-T cell therapies and continues to witness advancements in the field. Additionally, collaborations between academia, research institutes, and industry players in Europe contribute to the market’s growth.
Asia Pacific is poised to witness substantial growth in the coming years, primarily driven by the increasing prevalence of cancer and the growing demand for personalized medicine. Countries like China, Japan, and South Korea have made significant investments in biotechnology research and development, leading to the emergence of local players in the market. Moreover, the presence of a large patient population and the rising healthcare expenditure in the region contribute to market expansion.
Latin America and the Middle East and Africa regions are still in the early stages of adopting engineered T cell therapies. Limited access to healthcare resources, infrastructure challenges, and regulatory hurdles are some of the factors restraining market growth in these regions. However, improving healthcare infrastructure, rising awareness, and the efforts of government and private organizations to enhance cancer care are expected to drive market growth in the long term.
Competitive Landscape
Leading Companies in the Engineered T Cells Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Engineered T Cells market can be segmented based on therapy type, cancer type, and end-user.
Based on therapy type, the market can be divided into:
CAR-T cell therapy is the most widely adopted therapy type, accounting for the majority of the market share. This therapy involves modifying T cells to express chimeric antigen receptors that specifically target cancer cells. TCR-T cell therapy targets cancer cells by modifying T cells to express T-cell receptors that recognize specific antigens on cancer cells. TIL therapy, on the other hand, involves isolating tumor-infiltrating lymphocytes from a patient’s tumor and expanding them in the laboratory before reinfusing them back into the patient.
Based on cancer type, the market can be segmented into:
Hematological malignancies, such as leukemia and lymphoma, have been the primary focus of engineered T cell therapies. These cancers have shown significant response rates to CAR-T cell therapies, leading to their widespread adoption. However, there is increasing interest in expanding the application of engineered T cells to solid tumors, which present unique challenges due to their heterogeneity and immunosuppressive microenvironment.
Based on end-user, the market can be segmented into:
Hospitals and clinics are the primary end-users of engineered T cell therapies, where patients receive treatment under the supervision of healthcare professionals. Research institutes play a crucial role in advancing the field through preclinical and clinical research, exploring new therapeutic targets, and optimizing manufacturing processes. Biopharmaceutical companies are involved in the development, manufacturing, and commercialization of engineered T cell therapies.
Category-wise Insights
Each category within the Engineered T Cells market has its unique advantages and challenges. CAR-T cell therapy has demonstrated exceptional efficacy in certain hematological malignancies, but it requires complex manufacturing processes and can be associated with severe side effects. TCR-T cell therapy offers the potential to target a broader range of cancer types but requires further optimization and validation. TIL therapy presents an approach to harnessing the patient’s immune response but requires surgical intervention for tumor sample collection.
Key Benefits for Industry Participants and Stakeholders
The Engineered T Cells market offers several key benefits for industry participants and stakeholders:
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis provides a comprehensive assessment of the Engineered T Cells market:
Strengths:
Weaknesses:
Opportunities:
Threats:
Industry participants can leverage their strengths in efficacy, research and development capabilities, and collaborations to capitalize on opportunities such as expanding market applications, developing off-the-shelf products, and exploring combination therapies. Addressing weaknesses such as manufacturing costs and side effects is crucial to ensure market sustainability. Additionally, industry participants must navigate regulatory challenges, manage competition, and adapt to the evolving reimbursement landscape to mitigate threats and maintain a competitive advantage.
Market Key Trends
The Engineered T Cells market is influenced by several key trends that shape its growth and development:
Covid-19 Impact
The COVID-19 pandemic has had both positive and negative impacts on the Engineered T Cells market:
Positive Impact:
Negative Impact:
Despite the challenges, the long-term impact of the pandemic on the Engineered T Cells market is expected to be positive. The increased awareness of immunotherapies, advancements in research, and the resilience of the biopharmaceutical industry are likely to drive market growth in the post-pandemic period.
Key Industry Developments
The Engineered T Cells market has witnessed several key industry developments that shape its progress and market landscape:
Analyst Suggestions
Based on market trends and developments, analysts make several suggestions for industry participants in the Engineered T Cells market:
Future Outlook
The future of the Engineered T Cells market is promising, with significant growth opportunities on the horizon. The market is expected to witness continued technological advancements, expanding applications, and increasing adoption of engineered T cell therapies in clinical practice.
Advancements in gene editing technologies, such as CRISPR-Cas9, will continue to drive innovation in the field. These technologies will enable precise modifications in T cells, improve safety profiles, and broaden the applicability of engineered T cell therapies to different cancer types and treatment settings.
The expansion of engineered T cell therapies to solid tumors represents a significant growth opportunity. Ongoing research and clinical trials are exploring strategies to overcome the challenges associated with targeting solid tumors, including the immunosuppressive tumor microenvironment and heterogeneity of cancer cells.
Off-the-shelf engineered T cell therapies hold the potential to revolutionize the field by eliminating the need for patient-specific customization. The development of universal or allogeneic therapies would improve accessibility, reduce manufacturing costs, and streamline treatment processes.
Combination therapies, biomarker research, and patient selection strategies will be key areas of focus to optimize treatment outcomes and enhance the overall efficacy of engineered T cell therapies. The integration of engineered T cells with other treatment modalities, such as immune checkpoint inhibitors or targeted therapies, will offer synergistic effects and improved therapeutic responses.
As the regulatory landscape continues to evolve, industry participants should proactively engage with regulatory agencies and ensure compliance with the stringent requirements for approval and commercialization. Collaboration among industry players, research institutions, and regulatory authorities will be crucial for the development of robust clinical data, regulatory guidelines, and safety monitoring.
In terms of market access and affordability, industry participants should work towards cost-effective manufacturing processes, explore innovative reimbursement models, and advocate for insurance coverage to improve patient access to engineered T cell therapies.
Overall, the Engineered T Cells market is poised for significant growth and innovation in the coming years. The continuous advancements in technology, increasing understanding of cancer biology, and collaborative efforts across stakeholders will drive the market forward, providing new hope for patients battling cancer.
Conclusion
The Engineered T Cells market is experiencing rapid growth and represents a transformative approach to cancer treatment. Engineered T cell therapies offer personalized and targeted treatment options that harness the patient’s own immune system to recognize and eliminate cancer cells. The market is driven by factors such as increasing cancer prevalence, demand for more effective therapies, and technological advancements in cell engineering.
While the market presents significant opportunities, challenges remain, including high manufacturing costs, potential side effects, regulatory hurdles, and limited accessibility. However, industry participants can overcome these challenges through process optimization, strategic collaborations, focus on safety and efficacy, and addressing affordability and accessibility concerns.
What is Engineered T Cells?
Engineered T Cells are modified immune cells designed to enhance the body’s ability to fight diseases, particularly cancer. These cells are genetically altered to improve their targeting and killing capabilities against specific tumor cells.
What are the key players in the Engineered T Cells market?
Key players in the Engineered T Cells market include Novartis, Gilead Sciences, and Bristol-Myers Squibb, among others. These companies are at the forefront of developing innovative therapies and treatments utilizing engineered T cells.
What are the main drivers of the Engineered T Cells market?
The main drivers of the Engineered T Cells market include the increasing prevalence of cancer, advancements in genetic engineering technologies, and a growing focus on personalized medicine. These factors contribute to the rising demand for effective cancer therapies.
What challenges does the Engineered T Cells market face?
The Engineered T Cells market faces challenges such as high treatment costs, regulatory hurdles, and potential side effects associated with therapies. These factors can limit patient access and slow down market growth.
What opportunities exist in the Engineered T Cells market?
Opportunities in the Engineered T Cells market include the development of new therapies for various cancers, expansion into autoimmune diseases, and collaborations between biotech firms and research institutions. These avenues can lead to innovative treatment options.
What trends are shaping the Engineered T Cells market?
Trends shaping the Engineered T Cells market include the rise of combination therapies, advancements in CAR-T cell technology, and increased investment in research and development. These trends are driving innovation and improving patient outcomes.
Engineered T Cells market
| Segmentation Details | Description |
|---|---|
| Product Type | CAR T Cells, TCR T Cells, NK Cells, Others |
| Therapy Area | Oncology, Autoimmune Disorders, Infectious Diseases, Hematological Malignancies |
| End User | Hospitals, Research Institutes, Clinics, Contract Research Organizations |
| Delivery Mode | Intravenous, Subcutaneous, Intra-arterial, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Engineered T Cells Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at