444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The electronic clinical outcome assessment (eCOA) solutions market has experienced significant growth in recent years. As technology continues to advance, the healthcare industry is increasingly adopting digital tools to improve the collection, management, and analysis of patient-reported outcomes. eCOA solutions encompass a range of electronic platforms, such as mobile devices, tablets, and web-based applications, which allow patients to report their symptoms, treatment outcomes, and overall quality of life directly to healthcare providers.
Meaning
Electronic Clinical Outcome Assessment (eCOA) refers to the use of electronic devices or platforms to capture patient-reported outcomes in clinical trials, observational studies, and routine clinical practice. These outcomes include various subjective measures such as symptoms, treatment satisfaction, health-related quality of life, and adherence to treatment regimens. By utilizing eCOA solutions, healthcare professionals can obtain accurate and timely patient data, improving the efficiency and reliability of clinical assessments.
Executive Summary
The electronic clinical outcome assessment solutions market is poised for substantial growth in the coming years. With the increasing focus on patient-centered care, regulatory authorities’ endorsement of eCOA solutions, and the growing demand for efficient data collection and analysis, the market is witnessing a surge in adoption. The COVID-19 pandemic has further accelerated the adoption of remote data collection methods, driving the market’s expansion.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The eCOA solutions market is driven by a combination of factors, including regulatory guidelines, technological advancements, and the need for improved patient engagement. The market is highly competitive, with numerous vendors offering a wide range of eCOA platforms. The increasing emphasis on patient-reported outcomes in clinical trials and healthcare decision-making processes further fuels the market’s growth. However, challenges such as data security concerns, integration issues, and resistance to change hinder the market’s full potential.
Regional Analysis
The eCOA solutions market is segmented into several regions, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America currently holds the largest market share, driven by the presence of major eCOA solution providers, favorable regulatory policies, and advanced healthcare infrastructure. However, the Asia Pacific region is expected to witness significant growth due to the increasing adoption of digital health technologies, rising healthcare expenditure, and a growing focus on clinical research.
Competitive Landscape
Leading Companies in the Electronic Clinical Outcome Assessment Solutions Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

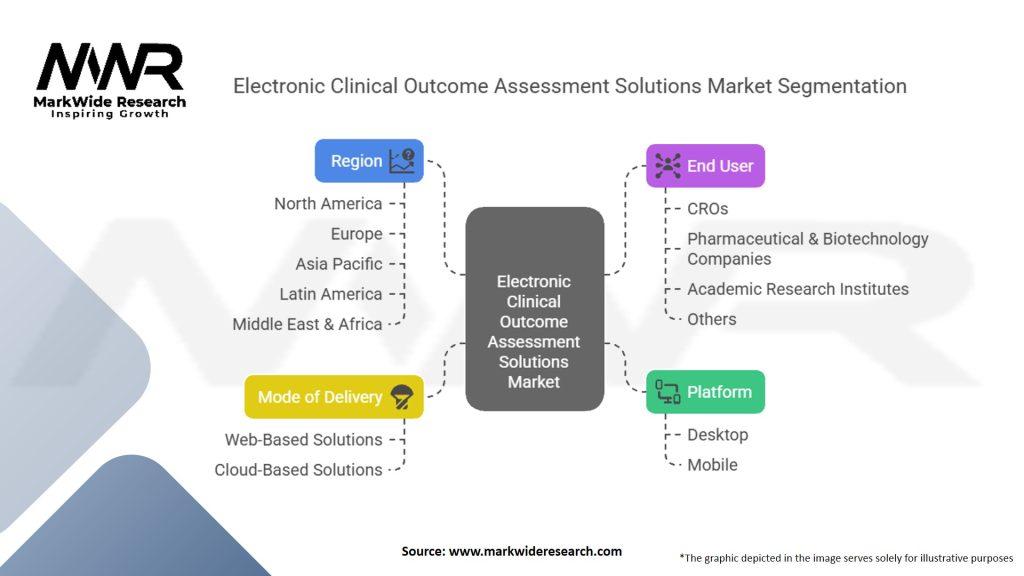
Segmentation
The eCOA solutions market can be segmented based on the type of platform, end-user, and therapeutic area. Platforms include mobile devices, tablets, web-based applications, and others. End-users encompass pharmaceutical companies, contract research organizations (CROs), hospitals and clinics, and academic research institutes. Therapeutic areas include oncology, cardiovascular diseases, central nervous system disorders, and others.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has significantly influenced the eCOA solutions market. The need for remote patient monitoring and data collection has surged, leading to increased adoption of eCOA platforms. Virtual clinical trials and telemedicine have become more prevalent, necessitating digital tools for data capture and patient-reported outcomes. The pandemic has highlighted the advantages of eCOA solutions, such as reduced patient visits, improved patient safety, and enhanced trial efficiency.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the eCOA solutions market looks promising, with a strong emphasis on patient-centric care and the growing demand for real-world evidence. Advancements in technology, including wearables, AI, and data analytics, will further enhance the capabilities of eCOA platforms. The market is expected to witness continued growth as more healthcare providers and pharmaceutical companies recognize the value of eCOA solutions in improving patient outcomes, streamlining clinical trials, and driving evidence-based decision-making.
Conclusion
The electronic clinical outcome assessment solutions market is experiencing significant growth, driven by the need for accurate and real-time patient-reported outcomes. eCOA platforms offer benefits such as improved data accuracy, enhanced patient engagement, and streamlined data collection. While challenges remain, such as data security concerns and resistance to change, the market presents opportunities for innovation and collaboration. With advancements in technology and a focus on patient-centric care, the future of the eCOA solutions market looks promising, with continued growth and adoption expected in the coming years.
What are Electronic Clinical Outcome Assessment Solutions?
Electronic Clinical Outcome Assessment Solutions refer to digital tools and platforms used to collect, analyze, and report patient-reported outcomes and clinical data in a streamlined manner. These solutions enhance the efficiency of clinical trials and improve patient engagement by providing real-time feedback and data collection.
Who are the key players in the Electronic Clinical Outcome Assessment Solutions Market?
Key players in the Electronic Clinical Outcome Assessment Solutions Market include Medidata Solutions, Oracle Corporation, and ERT, among others. These companies provide a range of software and services that facilitate electronic data capture and patient engagement in clinical trials.
What are the main drivers of growth in the Electronic Clinical Outcome Assessment Solutions Market?
The growth of the Electronic Clinical Outcome Assessment Solutions Market is driven by the increasing demand for efficient clinical trial processes, the rise in patient-centric approaches, and advancements in technology that enable real-time data collection and analysis.
What challenges does the Electronic Clinical Outcome Assessment Solutions Market face?
Challenges in the Electronic Clinical Outcome Assessment Solutions Market include concerns over data privacy and security, the need for regulatory compliance, and the potential for technology adoption barriers among healthcare professionals and patients.
What opportunities exist in the Electronic Clinical Outcome Assessment Solutions Market?
Opportunities in the Electronic Clinical Outcome Assessment Solutions Market include the expansion of telehealth services, the integration of artificial intelligence for data analysis, and the growing emphasis on personalized medicine, which requires robust outcome assessment tools.
What trends are shaping the Electronic Clinical Outcome Assessment Solutions Market?
Trends in the Electronic Clinical Outcome Assessment Solutions Market include the increasing use of mobile health applications, the shift towards decentralized clinical trials, and the incorporation of real-world evidence in clinical assessments, which enhance the relevance and applicability of clinical outcomes.
Electronic Clinical Outcome Assessment Solutions Market
| Segmentation Details | Information |
|---|---|
| Platform | Desktop, Mobile |
| End User | Contract Research Organizations (CROs), Pharmaceutical & Biotechnology Companies, Academic Research Institutes, Others |
| Mode of Delivery | Web-Based Solutions, Cloud-Based Solutions |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Electronic Clinical Outcome Assessment Solutions Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at