444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The Diagnostic Nuclear Drug Market is a vital segment of the healthcare industry, playing a crucial role in diagnostic imaging and nuclear medicine procedures. Diagnostic nuclear drugs, also known as radiopharmaceuticals, are radioactive compounds used in diagnostic imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) to visualize and evaluate physiological functions, metabolic processes, and organ structures within the human body. These drugs are essential for diagnosing various medical conditions, including cancer, cardiovascular diseases, neurological disorders, and musculoskeletal injuries.
Meaning:
Diagnostic nuclear drugs are pharmaceutical compounds containing radioactive isotopes, such as technetium-99m, fluorine-18, and iodine-123, that emit gamma or positron radiation. These drugs are administered to patients either intravenously, orally, or by inhalation and accumulate in specific tissues, organs, or biological targets of interest. By detecting the radiation emitted from these radioactive tracers, diagnostic imaging modalities such as PET and SPECT provide detailed anatomical and functional information for disease diagnosis, staging, and treatment planning.
Executive Summary:
The Diagnostic Nuclear Drug Market is experiencing steady growth due to the increasing prevalence of chronic diseases, rising demand for early and accurate diagnostic techniques, and advancements in nuclear medicine imaging technologies. Diagnostic nuclear drugs play a pivotal role in non-invasive diagnostic imaging procedures, offering healthcare providers valuable insights into disease pathology, treatment response, and patient management. However, market growth may be hampered by challenges such as regulatory hurdles, supply chain disruptions, and reimbursement issues.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Diagnostic Nuclear Drug Market operates in a dynamic environment influenced by factors such as technological innovations, regulatory developments, healthcare policies, and market competition. Market players must navigate these dynamics by investing in research and development, regulatory compliance, market access strategies, and stakeholder engagement to capitalize on growth opportunities and address market challenges effectively.
Regional Analysis:
The Diagnostic Nuclear Drug Market exhibits regional variations in terms of market size, growth potential, regulatory frameworks, and healthcare infrastructure. Key regions driving market growth include North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. Factors such as healthcare expenditure, regulatory landscape, reimbursement policies, and disease burden influence regional market dynamics and strategic decision-making for market players.
Competitive Landscape:
Leading Companies in the Diagnostic Nuclear Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
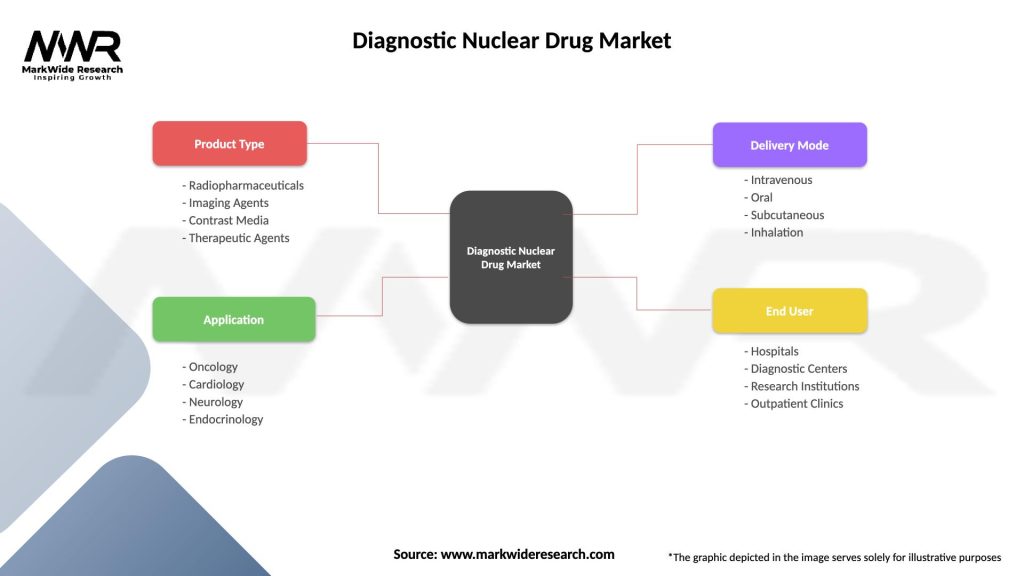
Segmentation:
The Diagnostic Nuclear Drug Market can be segmented based on various factors such as:
Segmentation provides insights into market trends, customer preferences, and growth opportunities, enabling market players to tailor their strategies and offerings to specific market segments and customer needs.
Category-wise Insights:
Key Benefits for Industry Participants and Stakeholders:
SWOT Analysis:
A SWOT analysis provides insights into the strengths, weaknesses, opportunities, and threats facing the Diagnostic Nuclear Drug Market:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has had both positive and negative effects on the Diagnostic Nuclear Drug Market. While the initial phase of the pandemic led to disruptions in elective procedures, reduced patient visits, and constraints on imaging services, the gradual recovery of healthcare systems, adoption of safety protocols, and resumption of diagnostic procedures have contributed to market rebound and growth opportunities. Telemedicine, remote monitoring, and decentralized imaging services have emerged as strategies to mitigate pandemic-related challenges and ensure continuity of care for patients requiring nuclear medicine procedures.
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The Diagnostic Nuclear Drug Market is poised for significant growth and innovation, driven by factors such as the increasing prevalence of chronic diseases, technological advancements in nuclear imaging, and the growing demand for personalized medicine and precision diagnostics. Market players will continue to focus on research and development, regulatory compliance, market access strategies, and collaborative partnerships to capitalize on growth opportunities and address evolving market dynamics effectively.
Conclusion:
In conclusion, the Diagnostic Nuclear Drug Market is a dynamic and evolving segment of the healthcare industry, offering essential diagnostic imaging solutions for a wide range of medical conditions. Despite challenges such as regulatory complexity, supply chain vulnerabilities, and reimbursement issues, the market presents significant opportunities for innovation, growth, and market expansion. By investing in research, technology, regulatory compliance, and stakeholder engagement, market participants can navigate challenges, capitalize on opportunities, and contribute to advancements in nuclear medicine and patient care globally.
What is Diagnostic Nuclear Drug?
Diagnostic Nuclear Drugs are specialized pharmaceutical compounds used in nuclear medicine to diagnose various medical conditions. They work by emitting radiation that can be detected by imaging devices, allowing healthcare professionals to visualize and assess organ function and disease presence.
What are the key players in the Diagnostic Nuclear Drug Market?
Key players in the Diagnostic Nuclear Drug Market include companies such as GE Healthcare, Siemens Healthineers, and Bracco Imaging, which are known for their innovative imaging technologies and radiopharmaceuticals, among others.
What are the growth factors driving the Diagnostic Nuclear Drug Market?
The growth of the Diagnostic Nuclear Drug Market is driven by factors such as the increasing prevalence of chronic diseases, advancements in imaging technologies, and the rising demand for early diagnosis and personalized medicine.
What challenges does the Diagnostic Nuclear Drug Market face?
The Diagnostic Nuclear Drug Market faces challenges including regulatory hurdles, high production costs, and the need for specialized training for healthcare professionals in handling radioactive materials.
What opportunities exist in the Diagnostic Nuclear Drug Market?
Opportunities in the Diagnostic Nuclear Drug Market include the development of new radiopharmaceuticals, expansion into emerging markets, and the integration of artificial intelligence in imaging processes to enhance diagnostic accuracy.
What trends are shaping the Diagnostic Nuclear Drug Market?
Trends in the Diagnostic Nuclear Drug Market include the increasing use of hybrid imaging techniques, such as PET/CT and SPECT/CT, and a growing focus on patient safety and sustainability in the production of radiopharmaceuticals.
Diagnostic Nuclear Drug Market
| Segmentation Details | Description |
|---|---|
| Product Type | Radiopharmaceuticals, Imaging Agents, Contrast Media, Therapeutic Agents |
| Application | Oncology, Cardiology, Neurology, Endocrinology |
| Delivery Mode | Intravenous, Oral, Subcutaneous, Inhalation |
| End User | Hospitals, Diagnostic Centers, Research Institutions, Outpatient Clinics |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Diagnostic Nuclear Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at