444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
Developmental and Epileptic Encephalopathies (DEE) is a group of rare and severe epileptic disorders characterized by early onset seizures, developmental delays, and cognitive impairment. These disorders significantly impact the quality of life of patients and their families, requiring specialized treatments and care. The DEE treatment market encompasses various pharmaceutical and non-pharmaceutical interventions aimed at managing the symptoms and improving the outcomes for individuals affected by these conditions. This market has witnessed significant growth in recent years due to increased awareness, advancements in medical research, and the growing demand for effective treatment options.
Meaning
Developmental and Epileptic Encephalopathies (DEE) refer to a group of neurological disorders that are characterized by both epilepsy and cognitive impairments. These conditions typically manifest in early infancy or childhood and can have a profound impact on the development and overall well-being of the affected individuals. DEE is often caused by genetic mutations or structural abnormalities in the brain, leading to frequent and severe seizures that are difficult to control. The cognitive impairments associated with DEE can range from mild to severe, affecting various aspects of intellectual functioning, including learning, language, and motor skills.
Executive Summary
The DEE treatment market has experienced significant growth in recent years, driven by advancements in medical research, increased awareness, and a growing number of individuals being diagnosed with these conditions. The market offers a wide range of treatment options, including antiepileptic medications, ketogenic diet therapy, neurostimulation devices, and supportive therapies. The primary goal of DEE treatment is to control seizures, improve cognitive function, and enhance the overall quality of life for patients. While there is no cure for DEE, ongoing research and development efforts are focused on discovering new therapeutic approaches and interventions.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
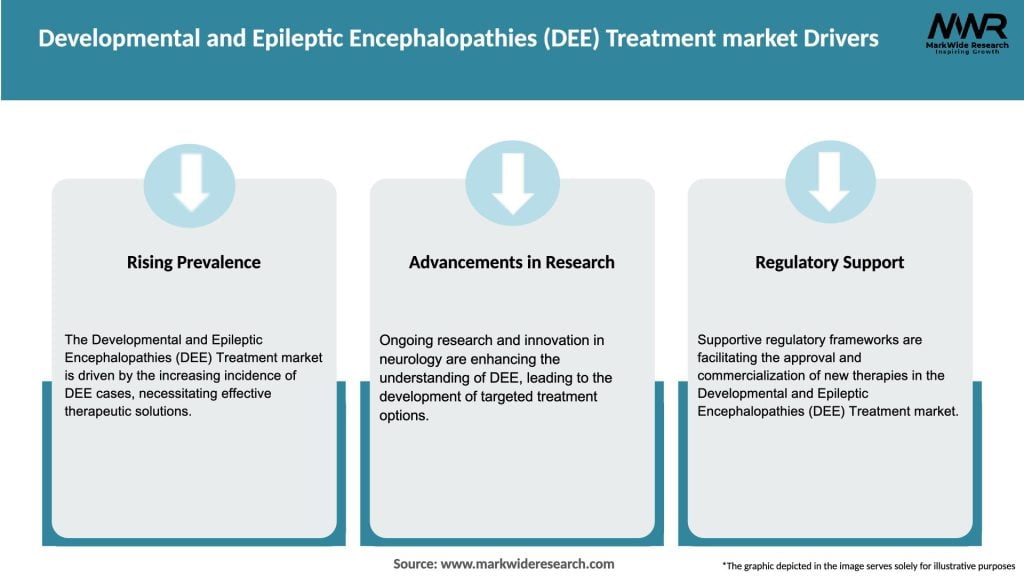
Market Dynamics
The DEE treatment market is dynamic and evolving, driven by various factors such as technological advancements, research breakthroughs, regulatory changes, and patient needs. The market is highly competitive, with several pharmaceutical companies, biotechnology firms, and medical device manufacturers actively involved in the development and commercialization of DEE treatment options. Additionally, collaborations between industry players, research institutions, and patient advocacy groups are shaping the market landscape and driving innovation. The market dynamics are influenced by regional variations in disease prevalence, healthcare infrastructure, reimbursement policies, and regulatory frameworks. As research continues to uncover new insights into the underlying causes and mechanisms of DEE, the market is expected to witness further growth and diversification.
Regional Analysis
The DEE treatment market exhibits regional variations in terms of disease prevalence, healthcare infrastructure, and treatment practices. Developed regions, such as North America and Europe, have well-established healthcare systems, advanced diagnostic capabilities, and a higher level of awareness about DEE. These regions have witnessed significant market growth, driven by increased diagnosis rates, access to specialized care, and advancements in treatment options. Developing regions, such as Asia-Pacific and Latin America, are experiencing a growing burden of DEE, driven by population growth, improved healthcare access, and rising awareness. However, these regions face challenges related to healthcare infrastructure, resource constraints, and limited access to specialized care. Nonetheless, efforts are being made to improve awareness, diagnosis, and treatment options in these regions, presenting opportunities for market expansion.
Competitive Landscape
Leading Companies in the Developmental and Epileptic Encephalopathies (DEE) Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
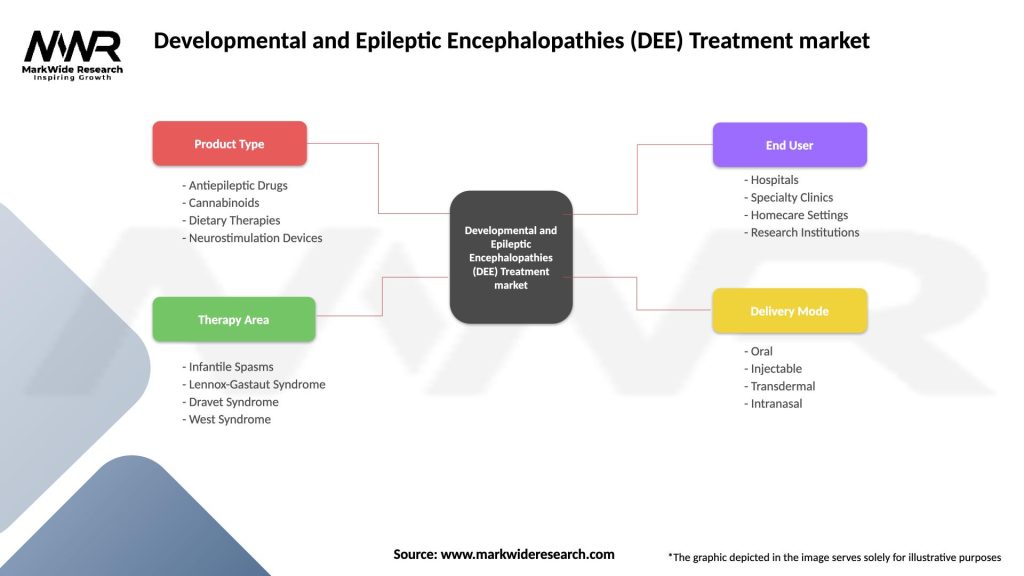
Segmentation
The DEE treatment market can be segmented based on various factors, including treatment type, distribution channel, and geography.
Based on Treatment Type, the market can be segmented into:
Based on Distribution Channel, the market can be segmented into:
Based on Geography, the market can be segmented into:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the DEE treatment market. The healthcare infrastructure in many regions was strained due to the overwhelming burden of the pandemic, leading to disruptions in routine care and delays in diagnosis and treatment for DEE patients. Additionally, access to specialized care and therapies was limited due to restrictions on non-essential healthcare services and concerns about virus transmission.
However, the pandemic also highlighted the importance of digital health technologies and telemedicine in managing DEE. Telehealth platforms allowed healthcare providers to remotely monitor patients, provide consultations, and adjust treatment plans when necessary. The pandemic accelerated the adoption of these technologies, improving access to care for individuals with DEE.
Moreover, the pharmaceutical and biotechnology industries continued their research and development activities despite the challenges posed by the pandemic. The ongoing efforts to develop new therapies and advance personalized medicine approaches were not halted, ensuring continued progress in the DEE treatment market.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the DEE treatment market holds promise with ongoing advancements in research, personalized medicine approaches, and the development of novel therapies. The expansion of digital health technologies and telemedicine is expected to improve access to care and enhance patient engagement. Furthermore, collaborations between industry players, research institutions, and patient advocacy groups will continue to drive innovation and accelerate the development of effective treatment options. With a growing emphasis on precision medicine, genetic research, and supportive therapies, the DEE treatment market is poised for significant advancements in the coming years.
Conclusion
The DEE treatment market has experienced substantial growth in recent years, driven by increased awareness, advancements in medical research, and the rising prevalence of these rare and severe epileptic disorders. The market offers a wide range of treatment options, including pharmaceutical interventions, dietary therapies, neurostimulation devices, and supportive therapies. Despite challenges related to limited treatment options, high costs, and diagnostic difficulties, the market presents opportunities for personalized medicine, development of novel therapies, and expansion of supportive interventions. Continued investment in research and development, collaborations, and a patient-centric approach are crucial for advancing the DEE treatment market. With ongoing advancements in technology, increasing awareness, and a focus on precision medicine, the future outlook for DEE treatment holds promise for improved outcomes and enhanced quality of life for individuals affected by these conditions.
What is Developmental and Epileptic Encephalopathies (DEE) Treatment?
Developmental and Epileptic Encephalopathies (DEE) Treatment refers to medical interventions aimed at managing severe neurological disorders characterized by developmental delays and intractable seizures. These treatments may include pharmacological therapies, dietary modifications, and surgical options tailored to individual patient needs.
What are the key players in the Developmental and Epileptic Encephalopathies (DEE) Treatment market?
Key players in the Developmental and Epileptic Encephalopathies (DEE) Treatment market include companies like UCB, Zogenix, and GW Pharmaceuticals, which are known for their innovative therapies and research in this field, among others.
What are the growth factors driving the Developmental and Epileptic Encephalopathies (DEE) Treatment market?
The growth of the Developmental and Epileptic Encephalopathies (DEE) Treatment market is driven by increasing awareness of these conditions, advancements in drug development, and a growing patient population seeking effective treatment options.
What challenges does the Developmental and Epileptic Encephalopathies (DEE) Treatment market face?
The Developmental and Epileptic Encephalopathies (DEE) Treatment market faces challenges such as high treatment costs, limited access to specialized care, and the complexity of accurately diagnosing various forms of DEE.
What future opportunities exist in the Developmental and Epileptic Encephalopathies (DEE) Treatment market?
Future opportunities in the Developmental and Epileptic Encephalopathies (DEE) Treatment market include the development of personalized medicine approaches, increased investment in research and development, and the potential for novel therapies targeting specific genetic mutations.
What trends are emerging in the Developmental and Epileptic Encephalopathies (DEE) Treatment market?
Emerging trends in the Developmental and Epileptic Encephalopathies (DEE) Treatment market include the rise of telemedicine for patient management, the integration of digital health technologies, and a focus on multidisciplinary care approaches to improve patient outcomes.
Developmental and Epileptic Encephalopathies (DEE) Treatment market
| Segmentation Details | Description |
|---|---|
| Product Type | Antiepileptic Drugs, Cannabinoids, Dietary Therapies, Neurostimulation Devices |
| Therapy Area | Infantile Spasms, Lennox-Gastaut Syndrome, Dravet Syndrome, West Syndrome |
| End User | Hospitals, Specialty Clinics, Homecare Settings, Research Institutions |
| Delivery Mode | Oral, Injectable, Transdermal, Intranasal |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Developmental and Epileptic Encephalopathies (DEE) Treatment Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at