444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Cystoscopy Needles market serves a critical role in urological procedures, providing surgeons with precision tools for diagnostic and therapeutic cystoscopy procedures. These needles enable clinicians to access the urinary bladder, collect tissue samples, perform biopsies, and administer medications with accuracy and minimal patient discomfort.
Meaning
Cystoscopy needles are specialized medical instruments used in cystoscopy procedures to access the urinary bladder through the urethra. These needles facilitate tissue sampling, biopsy collection, lesion localization, and drug delivery, enabling clinicians to diagnose and treat various urinary tract conditions, including bladder cancer, urinary infections, and bladder wall abnormalities.
Executive Summary
The Cystoscopy Needles market has witnessed significant growth due to rising incidence rates of urological disorders, increasing demand for minimally invasive diagnostic techniques, and advancements in cystoscopic technologies. Key players in the market offer a diverse range of cystoscopy needle products designed to meet the evolving needs of urologists, enhance procedural efficiency, and improve patient outcomes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Cystoscopy Needles market operates within a dynamic healthcare ecosystem shaped by demographic trends, technological innovations, regulatory reforms, and market forces that influence product development, market entry strategies, and competitive positioning among industry stakeholders.
Regional Analysis
Regional variations in healthcare infrastructure, reimbursement policies, disease epidemiology, and market dynamics influence demand patterns, adoption rates, and growth opportunities for cystoscopy needles across different geographic regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East.
Competitive Landscape
Leading Companies in Cystoscopy Needles Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
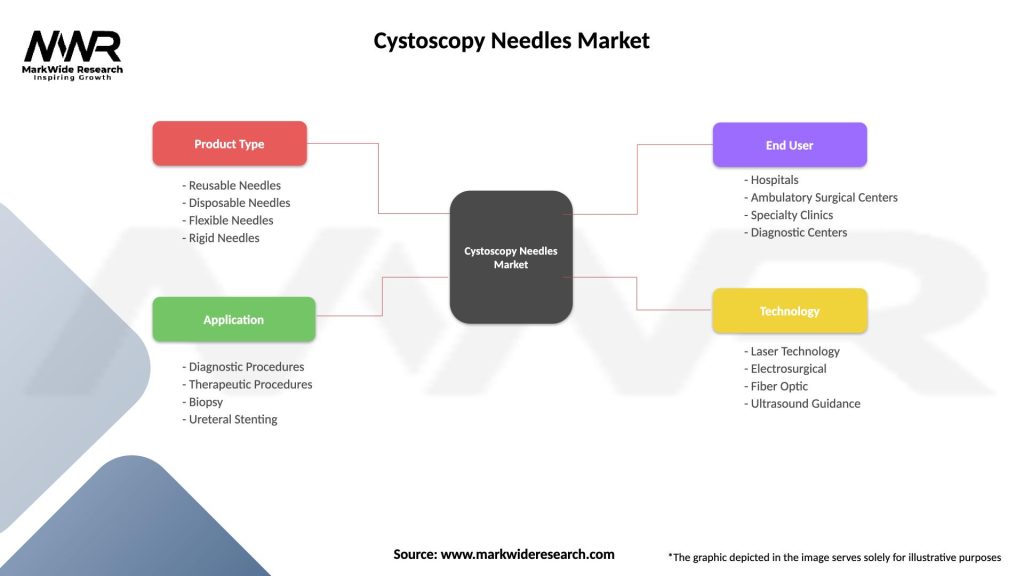
Segmentation
The Cystoscopy Needles market can be segmented based on needle type (fine needle biopsy, core biopsy, aspiration needle), application (diagnostic cystoscopy, therapeutic cystoscopy), end-user (hospitals, ambulatory surgical centers, specialty clinics), and geographic region (North America, Europe, Asia-Pacific, Latin America, Middle East, Africa).
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
Key Industry Developments
Analyst Suggestions
Future Outlook
The Cystoscopy Needles market is poised for sustained growth driven by increasing demand for minimally invasive diagnostic techniques, rising prevalence of bladder cancer and urological disorders, and ongoing technological advancements in needle design, imaging modalities, and procedural guidance systems. Key trends such as personalized medicine, digital health integration, and telemedicine adoption will shape the future landscape of cystoscopy practice, fostering innovation, collaboration, and patient-centered care delivery models in the evolving urology market.
Conclusion
The Cystoscopy Needles market plays a pivotal role in urological practice, enabling clinicians to perform precise diagnostic and therapeutic interventions for patients with bladder cancer, urinary tract infections, and other urological disorders. As the demand for minimally invasive diagnostic techniques and personalized treatment approaches continues to grow, opportunities abound for cystoscopy needle manufacturers, healthcare providers, and industry stakeholders to innovate, collaborate, and drive advancements in cystoscopy practice, improving patient outcomes, enhancing procedural efficiency, and advancing the standard of care in urology. By embracing technological innovation, fostering clinical excellence, and prioritizing patient-centered care principles, the cystoscopy needle market can navigate evolving healthcare trends, regulatory challenges, and market dynamics, contributing to the advancement of urological science and the improvement of patient lives worldwide.
What is Cystoscopy Needles?
Cystoscopy needles are specialized medical instruments used during cystoscopy procedures to access the bladder and urinary tract. They are designed for precision and safety in various urological applications, including biopsies and fluid drainage.
What are the key players in the Cystoscopy Needles Market?
Key players in the Cystoscopy Needles Market include Boston Scientific, Medtronic, and Cook Medical, among others. These companies are known for their innovative products and contributions to urological healthcare.
What are the growth factors driving the Cystoscopy Needles Market?
The Cystoscopy Needles Market is driven by factors such as the increasing prevalence of urological disorders, advancements in minimally invasive surgical techniques, and a growing aging population requiring urological interventions.
What challenges does the Cystoscopy Needles Market face?
Challenges in the Cystoscopy Needles Market include the high cost of advanced medical devices and the risk of complications associated with cystoscopy procedures. Additionally, regulatory hurdles can impact product development and market entry.
What opportunities exist in the Cystoscopy Needles Market?
Opportunities in the Cystoscopy Needles Market include the development of innovative needle designs and materials that enhance patient safety and comfort. There is also potential for growth in emerging markets as healthcare infrastructure improves.
What trends are shaping the Cystoscopy Needles Market?
Trends in the Cystoscopy Needles Market include the increasing adoption of robotic-assisted surgeries and the integration of advanced imaging technologies. These innovations aim to improve the accuracy and efficiency of cystoscopy procedures.
Cystoscopy Needles Market
| Segmentation Details | Description |
|---|---|
| Product Type | Reusable Needles, Disposable Needles, Flexible Needles, Rigid Needles |
| Application | Diagnostic Procedures, Therapeutic Procedures, Biopsy, Ureteral Stenting |
| End User | Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Diagnostic Centers |
| Technology | Laser Technology, Electrosurgical, Fiber Optic, Ultrasound Guidance |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at