444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The critical care diagnostic market is a rapidly growing sector within the healthcare industry that focuses on diagnosing and monitoring life-threatening conditions in patients requiring intensive care. This market encompasses a wide range of diagnostic tests, devices, and equipment used in critical care settings, such as intensive care units (ICUs), emergency departments, and operating rooms. The primary objective of critical care diagnostics is to enable healthcare professionals to make accurate and timely decisions for optimal patient care.
Critical care diagnostics refer to the tools and techniques used to assess the severity, progression, and treatment response of critical illnesses in patients. These diagnostics help healthcare providers monitor various parameters, such as vital signs, organ function, blood chemistry, and biomarkers, to evaluate a patient’s condition and guide appropriate interventions. The accurate and timely diagnosis provided by critical care diagnostics is crucial in improving patient outcomes and reducing morbidity and mortality rates in critically ill individuals.
Executive Summary
The critical care diagnostic market has witnessed significant growth in recent years, driven by factors such as the increasing prevalence of chronic diseases, technological advancements in diagnostic devices, and the rising demand for personalized medicine. This market offers a wide array of diagnostic solutions, including point-of-care testing (POCT) devices, laboratory tests, imaging modalities, and molecular diagnostics. The need for accurate and rapid diagnostic tools in critical care settings has fueled the adoption of these advanced technologies.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The critical care diagnostic market is dynamic and influenced by various factors. These include advancements in technology, changing healthcare landscapes, regulatory policies, and shifts in patient demographics. Market dynamics play a significant role in shaping the demand, supply, and competitive landscape of critical care diagnostics.
The market is driven by the need for accurate and timely diagnosis, increasing patient awareness about the benefits of early detection, and the growing focus on personalized medicine. On the other hand, challenges such as high costs, stringent regulations, and limited accessibility can impede market growth. Monitoring market dynamics and adapting strategies accordingly is crucial for market players to stay competitive and capitalize on emerging opportunities.
Regional Analysis
The critical care diagnostic market exhibits regional variations in terms of market size, growth rate, and adoption of diagnostic technologies. North America has traditionally been a dominant market, owing to well-established healthcare infrastructure, technological advancements, and high healthcare expenditure. Europe follows closely, with a strong emphasis on research and development in the field of diagnostics.
Asia Pacific is expected to witness rapid market growth, driven by a large population base, increasing prevalence of chronic diseases, and rising healthcare expenditure. The region offers significant growth potential for market players, particularly in countries like China and India, which are witnessing rapid urbanization and improvements in healthcare infrastructure.
Latin America, the Middle East, and Africa also present opportunities for market expansion, albeit with certain challenges related to accessibility, infrastructure limitations, and socioeconomic factors. However, increasing investments in healthcare and growing awareness about critical care diagnostics are contributing to the growth of these regions.
Competitive Landscape
Leading companies in the Critical Care Diagnostic market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
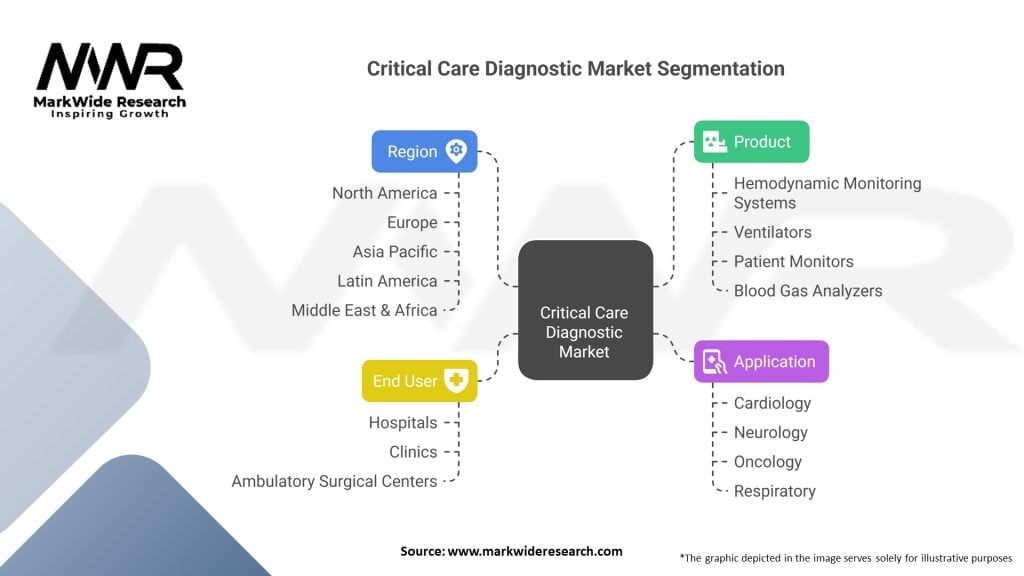
Segmentation
The critical care diagnostic market can be segmented based on various factors, including diagnostic technique, end-user, and region. Common segmentation categories include:
Segmentation allows for a focused analysis of specific market segments, enabling companies to tailor their strategies and offerings to the unique requirements of different customer groups.
Category-wise Insights
Laboratory testing is a significant category within the critical care diagnostic market. It involves the analysis of various specimens, such as blood, urine, and tissue samples, to detect and monitor critical illnesses. This category includes a wide range of tests, including blood chemistry analysis, microbiological cultures, coagulation studies, and genetic testing.
Laboratory testing plays a crucial role in diagnosing and monitoring conditions such as sepsis, organ dysfunction, metabolic disorders, and infectious diseases. These tests provide healthcare professionals with valuable insights into a patient’s health status, helping them make informed decisions regarding treatment and patient management.
Advancements in laboratory testing technologies have led to increased accuracy, efficiency, and automation. Automated analyzers and advanced algorithms have improved turnaround times, allowing for quicker results and faster decision-making. Moreover, the integration of molecular diagnostics and genetic testing has enabled the identification of specific genetic markers and mutations associated with critical illnesses, facilitating targeted therapies and personalized medicine approaches.
Laboratory testing is predominantly performed in hospitals, diagnostic laboratories, and specialized testing centers. These facilities are equipped with state-of-the-art equipment, skilled laboratory technicians, and quality assurance protocols to ensure accurate and reliable test results. Timely and accurate laboratory testing is essential for effective patient care, as it enables healthcare professionals to monitor disease progression, assess treatment response, and make critical decisions in emergency situations.
The laboratory testing category within the critical care diagnostic market is expected to experience substantial growth due to the increasing demand for accurate and comprehensive diagnostic services. Technological advancements, such as the integration of artificial intelligence (AI) and machine learning algorithms in laboratory analyzers, are further enhancing the accuracy and efficiency of these tests. Moreover, the rising prevalence of chronic diseases and infectious diseases, coupled with the aging population, is driving the need for advanced laboratory testing solutions.
Key players in the critical care diagnostic market are focusing on developing innovative laboratory testing platforms that offer rapid and accurate results, improved workflow efficiency, and seamless integration with electronic medical records (EMRs) and other healthcare systems. Strategic partnerships and collaborations between diagnostic companies and healthcare institutions are also fostering advancements in laboratory testing, enabling the development of new tests and the expansion of test menus.
In summary, laboratory testing is a vital category within the critical care diagnostic market, providing healthcare professionals with essential information for diagnosing and managing critical illnesses. Advancements in technology and the increasing demand for accurate and efficient diagnostic services are driving the growth of this category, with a focus on automation, integration of molecular diagnostics, and partnerships for innovation.
Key Benefits for Industry Participants and Stakeholders
The critical care diagnostic market offers several key benefits for industry participants and stakeholders, including:
SWOT Analysis
A SWOT analysis of the critical care diagnostic market provides insights into its strengths, weaknesses, opportunities, and threats:
By understanding these factors, industry participants can capitalize on strengths, address weaknesses, leverage opportunities, and mitigate threats to stay competitive and achieve sustainable growth in the critical care diagnostic market.
Market Key Trends
Several key trends are shaping the critical care diagnostic market:
COVID-19 Impact
The COVID-19 pandemic has significantly impacted the critical care diagnostic market. The need for accurate and rapid diagnostic tests for COVID-19 has surged, leading to a rapid development and adoption of diagnostic solutions. Point-of-care antigen and molecular tests have played a critical role in identifying infected individuals, monitoring disease progression, and guiding appropriate interventions.
The pandemic has also accelerated the adoption of telemedicine and remote monitoring technologies in critical care settings. These technologies enable virtual consultations, remote patient monitoring, and real-time data sharing, minimizing the risk of exposure and preserving healthcare resources.
Additionally, the COVID-19 pandemic has highlighted the importance of diagnostic supply chain resilience, global collaboration, and the need for robust testing infrastructure to effectively respond to infectious disease outbreaks in critical care settings.
Key Industry Developments
The critical care diagnostic market has witnessed several key industry developments:
Analyst Suggestions
Based on market trends and dynamics, analysts suggest the following strategies for industry participants in the critical care diagnostic market:
Future Outlook
The future of the critical care diagnostic market looks promising, with significant growth opportunities driven by technological advancements, increasing demand for personalized medicine, and the need for accurate and rapid diagnostic solutions in critical care settings. The market is expected to witness a shift towards integrated and connected diagnostic platforms, AI-driven diagnostics, and the adoption of remote monitoring technologies.
The ongoing focus on infectious disease diagnostics, as highlighted by the COVID-19 pandemic, is likely to continue. This will lead to the development of advanced testing solutions, improved testing infrastructure, and stronger collaborations between diagnostic companies and public health agencies.
Moreover, the integration of diagnostics with digital health platforms, telemedicine, and artificial intelligence is anticipated to reshape critical care diagnostics, enabling more efficient and effective patient care.
However, industry participants should be prepared to address challenges such as high costs, regulatory complexities, and limited accessibility in certain regions. Adapting strategies, investing in research and development, and leveraging partnerships will be key to capitalizing on emerging opportunities and maintaining a competitive edge in the evolving critical care diagnostic market.
Conclusion
The critical care diagnostic market is witnessing significant growth and transformation, driven by technological advancements, increasing demand for personalized medicine, and the need for accurate and rapid diagnostic solutions in critical care settings. Industry participants have the opportunity to shape the future of critical care diagnostics by focusing on innovation, collaborations, regulatory compliance, and customer-centric approaches.
What is Critical Care Diagnostic?
Critical Care Diagnostic refers to the medical processes and technologies used to assess and monitor patients in critical care settings. This includes various diagnostic tools and tests that help in the timely identification of life-threatening conditions.
What are the key players in the Critical Care Diagnostic market?
Key players in the Critical Care Diagnostic market include Abbott Laboratories, Siemens Healthineers, and Philips Healthcare, among others. These companies are known for their innovative diagnostic solutions and technologies that enhance patient care in critical settings.
What are the main drivers of growth in the Critical Care Diagnostic market?
The growth of the Critical Care Diagnostic market is driven by the increasing prevalence of chronic diseases, advancements in diagnostic technologies, and the rising demand for rapid diagnostic tests in emergency care. These factors contribute to improved patient outcomes and efficiency in critical care environments.
What challenges does the Critical Care Diagnostic market face?
The Critical Care Diagnostic market faces challenges such as high costs of advanced diagnostic equipment, regulatory hurdles, and the need for skilled personnel to operate complex diagnostic tools. These factors can limit accessibility and adoption in some healthcare settings.
What opportunities exist in the Critical Care Diagnostic market?
Opportunities in the Critical Care Diagnostic market include the development of point-of-care testing solutions, integration of artificial intelligence in diagnostics, and expansion into emerging markets. These trends can enhance the efficiency and effectiveness of critical care diagnostics.
What are the current trends in the Critical Care Diagnostic market?
Current trends in the Critical Care Diagnostic market include the increasing use of telemedicine for remote monitoring, the rise of portable diagnostic devices, and the focus on personalized medicine. These innovations aim to improve patient care and streamline diagnostic processes.
Critical Care Diagnostic Market:
| Segmentation | Details |
|---|---|
| Product | Hemodynamic Monitoring Systems, Ventilators, Patient Monitors, Blood Gas Analyzers, Others |
| Application | Cardiology, Neurology, Oncology, Respiratory, Others |
| End User | Hospitals, Clinics, Ambulatory Surgical Centers, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Critical Care Diagnostic market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at