444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The cervical cancer drugs market is a rapidly growing segment within the pharmaceutical industry. Cervical cancer is a type of cancer that affects the cervix, the lower part of the uterus. It is the fourth most common cancer in women worldwide and is a major cause of morbidity and mortality. The market for cervical cancer drugs includes various types of medications, such as chemotherapy drugs, targeted therapies, immunotherapies, and hormonal therapies, that are used for the treatment of this disease.
Meaning
Cervical cancer drugs refer to the pharmaceutical products and treatments used to manage and combat cervical cancer. These drugs are designed to target cancer cells, inhibit their growth, and prevent the spread of the disease. They may be administered through various routes, including oral, intravenous, and intramuscular, depending on the specific drug and treatment plan prescribed by healthcare professionals.
Executive Summary
The cervical cancer drugs market has witnessed significant growth in recent years, driven by increasing awareness about the disease, advancements in medical technology, and a rise in the global incidence of cervical cancer. The market is highly competitive, with several pharmaceutical companies actively involved in research and development to develop innovative drugs for the effective treatment of cervical cancer. The market is expected to continue its growth trajectory in the coming years, fueled by emerging economies and improved access to healthcare facilities.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Shift Toward Biologics: From 2018 to 2023, biologic and immunotherapeutic share of the market increased from 15% to 28%, reflecting higher uptake of bevacizumab and checkpoint inhibitors.
Combination Regimens: Trials combining pembrolizumab with chemotherapy ± bevacizumab report overall response rates (ORR) exceeding 60% in front-line recurrent/metastatic settings.
Antibody–Drug Conjugate Growth: Tisotumab vedotin achieved ORR of 24% in platinum-refractory patients, driving ADC segment CAGR >20%.
Emerging Market Surge: Asia Pacific exhibits the fastest regional CAGR (~8%), propelled by China’s expanded oncology reimbursement and India’s generic chemotherapeutic usage.
HPV Vaccination Impact: While preventive vaccines (Gardasil®, Cervarix®) reduce incidence long-term, current patient population still necessitates robust drug therapies.
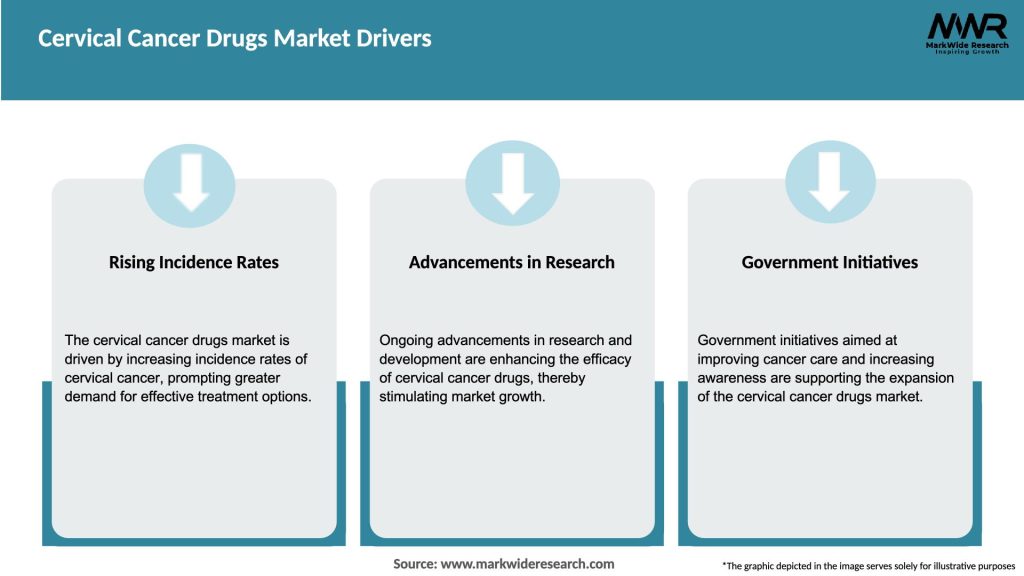
Market Drivers
Rising Disease Burden: Globally, over 600,000 new cervical cancer cases occur annually; projected to rise with aging populations and improved detection.
Advancements in Immunotherapy: Checkpoint inhibitors demonstrate durable responses, encouraging oncology communities to adopt immunomodulatory strategies.
Regulatory Accelerations: FDA’s Breakthrough Therapy designations and EMA PRIME schemes shorten approval timelines for promising agents.
Combination Strategies: Synergistic regimens combining anti-angiogenics, immunotherapies, and chemotherapy enhance outcomes and expand patient eligibility.
Healthcare Access Improvements: Expanded insurance coverage and oncology center proliferation in emerging markets increase patient access to novel therapies.
Market Restraints
High Treatment Costs: Biologics and ADCs often exceed USD 100,000 per treatment course, limiting access in low- and middle-income countries.
Adverse Event Profiles: Immune-related toxicities (e.g., colitis, pneumonitis) and ADC-specific side effects (e.g., ocular toxicity) complicate treatment.
Screening Gaps: Inadequate screening programs in some regions lead to late-stage presentations less amenable to targeted therapies.
Resistance Mechanisms: Tumor evasion via alternative angiogenic pathways and immune microenvironment suppression necessitates next-generation agents.
Reimbursement Hurdles: Payor reluctance to cover high-cost therapies without robust long-term survival data may delay market uptake.
Market Opportunities
Therapeutic HPV Vaccines: Late-stage trials of DNA- and vector-based vaccines targeting HPV oncoproteins E6/E7 show potential to eliminate residual disease and reduce recurrence.
Adoptive Cell Therapies: Early-phase CAR-T and TIL therapies directed at HPV antigens could deliver unprecedented response durability.
Biomarker-Guided Therapy: PD-L1 expression, tumor mutational burden, and immune signature profiling enable personalized immunotherapy approaches.
Oral Small Molecule Inhibitors: Development of PI3K, FGFR, and epigenetic inhibitors offers all-oral regimens that may improve patient convenience and adherence.
Digital Health Integration: Tele-oncology platforms and real-world data collection facilitate monitoring of treatment efficacy and safety, supporting payor negotiations.

Market Dynamics
Collaborative Trials: Multi-center, global Phase III studies assess combinations of pembrolizumab, durvalumab, and novel ADCs, accelerating clinical evidence generation.
M&A Activity: Large pharmaceutical firms acquiring biotech innovators (e.g., ADC specialists, vaccine developers) to bolster pipelines.
Generic Chemotherapy Competition: Low-cost cisplatin and paclitaxel generics exert pricing pressure on combination regimens, necessitating value-based pricing strategies for novel agents.
Public–Private Partnerships: Initiatives like the WHO’s Cervical Cancer Elimination program collaborate with pharma to improve drug access in low-resource settings.
Regulatory Harmonization: EMA and FDA aligning on accelerated pathways for oncology drugs reduces duplication and expedites global launch.
Regional Analysis
The cervical cancer drugs market can be analyzed across various regions, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America and Europe currently dominate the market due to well-established healthcare infrastructure, high awareness levels, and significant investments in cancer research. The Asia Pacific region is expected to witness rapid growth in the coming years, driven by increasing healthcare expenditure, rising awareness, and the presence of a large patient pool. Latin America, the Middle East, and Africa are also projected to experience growth due to improving healthcare facilities and the implementation of preventive programs.
Competitive Landscape
Leading Companies in the Cervical Cancer Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The cervical cancer drugs market can be segmented based on drug type, distribution channel, and region.
Based on drug type, the market can be segmented into:
Based on distribution channel, the market can be segmented into:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The global outbreak of COVID-19 has had a significant impact on the healthcare industry, including the cervical cancer drugs market. The pandemic led to disruptions in the healthcare infrastructure, diversion of resources towards managing COVID-19 cases, and changes in patient behavior, such as delayed or canceled screenings and treatments.
During the pandemic, healthcare systems faced challenges in providing timely access to cervical cancer drugs and maintaining continuity of care for patients. However, efforts were made to ensure the availability of essential medications and prioritize treatments for cancer patients.
The pandemic also highlighted the importance of telemedicine and remote patient monitoring, enabling healthcare professionals to provide consultations and follow-up care virtually. These technological advancements have the potential to reshape the delivery of cervical cancer drugs and improve patient access to healthcare services in the long term.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future outlook for the cervical cancer drugs market is optimistic, with the potential for significant growth. Advances in technology, increasing awareness, and collaborations between stakeholders are expected to drive innovation and the development of more effective therapies. The market will likely witness the entry of new drugs and treatment modalities, offering improved options for patients and healthcare providers. However, addressing challenges such as cost, access to healthcare, and regulatory hurdles will be crucial to ensure the widespread availability and affordability of cervical cancer drugs.
Conclusion
The cervical cancer drugs market is poised for growth, driven by increasing incidence rates, technological advancements, and growing healthcare expenditure. The market offers opportunities for pharmaceutical companies, healthcare providers, and patients to improve treatment outcomes and enhance patient care. However, challenges such as high treatment costs, limited access to healthcare facilities, and regulatory hurdles need to be addressed. With ongoing research and development efforts, collaborations, and a focus on personalized medicine, the future of the cervical cancer drugs market looks promising, paving the way for better management and treatment of this devastating disease.
What is Cervical Cancer Drugs?
Cervical Cancer Drugs refer to pharmaceutical treatments specifically designed to combat cervical cancer, which may include chemotherapy, targeted therapy, and immunotherapy options. These drugs aim to inhibit cancer cell growth and improve patient outcomes.
What are the key players in the Cervical Cancer Drugs Market?
Key players in the Cervical Cancer Drugs Market include Merck & Co., GlaxoSmithKline, Bristol-Myers Squibb, and Roche, among others. These companies are involved in the development and commercialization of innovative therapies for cervical cancer.
What are the growth factors driving the Cervical Cancer Drugs Market?
The growth of the Cervical Cancer Drugs Market is driven by increasing awareness of cervical cancer screening, advancements in drug development, and rising incidences of HPV infections. Additionally, supportive government initiatives for vaccination and treatment are contributing to market expansion.
What challenges does the Cervical Cancer Drugs Market face?
The Cervical Cancer Drugs Market faces challenges such as high treatment costs, stringent regulatory approvals, and the need for ongoing research to address drug resistance. These factors can hinder patient access to effective therapies.
What opportunities exist in the Cervical Cancer Drugs Market?
Opportunities in the Cervical Cancer Drugs Market include the development of personalized medicine approaches, increasing investment in research and development, and the potential for combination therapies. These factors may enhance treatment efficacy and patient outcomes.
What trends are shaping the Cervical Cancer Drugs Market?
Trends shaping the Cervical Cancer Drugs Market include the rise of immunotherapy, the integration of biomarker testing in treatment plans, and the focus on patient-centric care. These trends are influencing how therapies are developed and administered.
Cervical Cancer Drugs Market
| Segmentation Details | Details |
|---|---|
| Drug Type | Chemotherapy Drugs, Targeted Therapy Drugs, Immunotherapy Drugs, Hormone Therapy Drugs, Others |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Cervical Cancer Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at