444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Cervical cancer is a significant health concern globally, affecting thousands of women each year. Early detection and accurate diagnosis play a crucial role in improving treatment outcomes and reducing mortality rates. The cervical cancer diagnostic tests market encompasses various screening and diagnostic methods designed to identify cervical abnormalities and the presence of cancerous cells. This comprehensive market analysis aims to provide valuable insights into the current trends, key market drivers, restraints, and opportunities shaping the cervical cancer diagnostic tests market.
Cervical cancer diagnostic tests refer to the range of screening and diagnostic methods employed to detect abnormalities in the cervix and identify the presence of cervical cancer. These tests aid in the early detection of cervical cancer, allowing for timely treatment interventions. The market for cervical cancer diagnostic tests includes both traditional screening methods, such as Pap smear tests, as well as advanced technologies like HPV DNA testing, colposcopy, and biopsy.
Executive Summary
The executive summary provides a concise overview of the cervical cancer diagnostic tests market, highlighting the key findings and market insights. It summarizes the market’s size, growth rate, and major market players, setting the stage for a deeper exploration of the market dynamics and key trends.
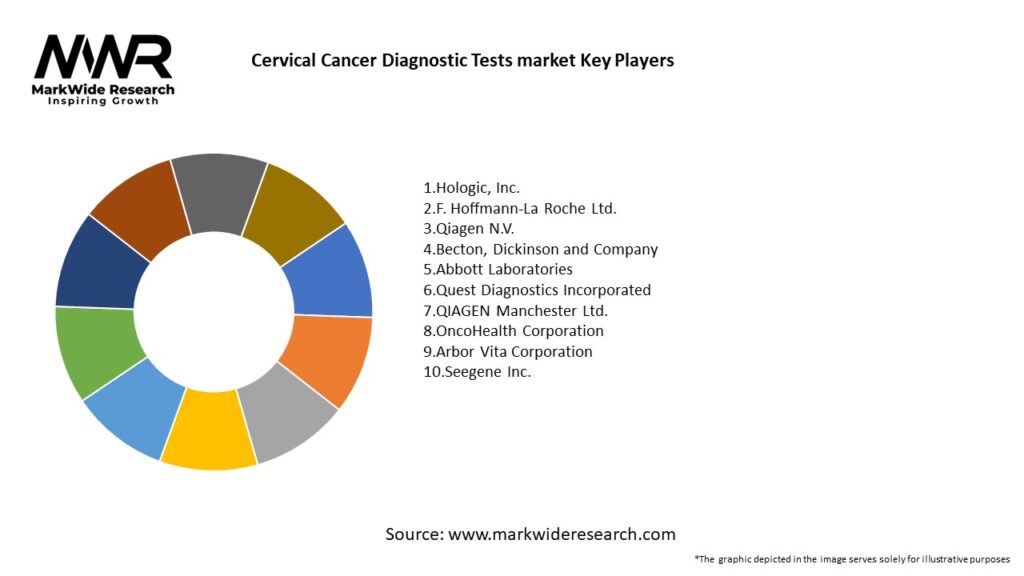
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
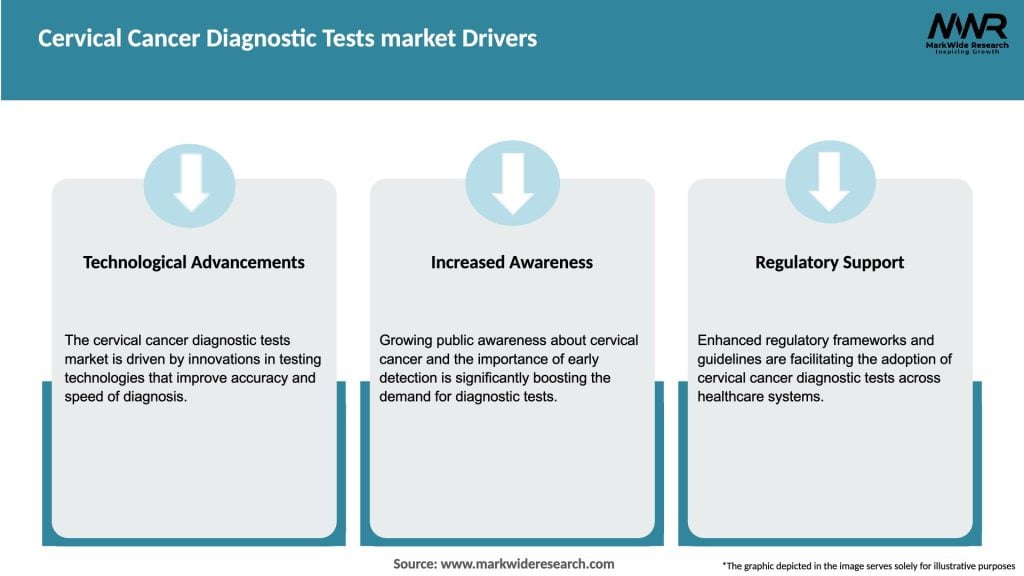
Market Drivers
Several factors are driving the growth of the Cervical Cancer Diagnostic Tests market:
Market Restraints
Despite its growth prospects, the Cervical Cancer Diagnostic Tests market faces several challenges:
Market Opportunities
The Cervical Cancer Diagnostic Tests market presents several growth opportunities:
Market Dynamics
The Europe Cervical Cancer Diagnostic Tests market is influenced by various factors that shape the direction of its growth:
Regional Analysis
The Cervical Cancer Diagnostic Tests market in Europe is showing varied growth patterns across different regions:
Competitive Landscape
Leading companies in the Cervical Cancer Diagnostic Tests market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
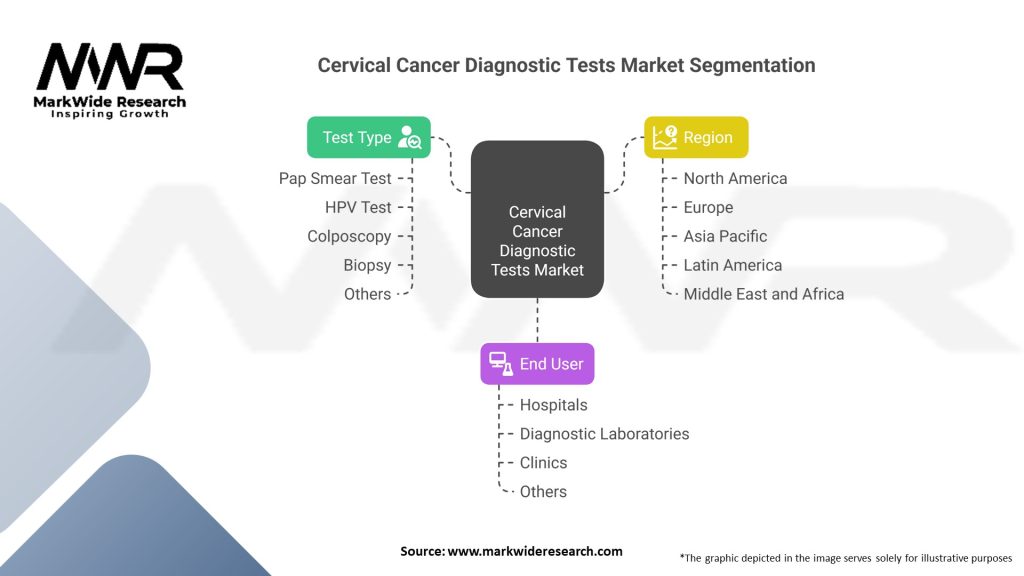
Segmentation
The Cervical Cancer Diagnostic Tests market can be segmented based on the following factors:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
The Cervical Cancer Diagnostic Tests market offers numerous benefits to participants and stakeholders:
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has disrupted routine healthcare services, including cervical cancer screenings. However, it has also accelerated the adoption of remote and non-invasive testing methods, allowing patients to continue screenings during lockdowns. Post-pandemic, there is a renewed focus on improving access to screening and diagnostic services to address the backlog caused by the pandemic.
Key Industry Developments
Analyst Suggestions
Industry analysts recommend:
Future Outlook
The future outlook section provides a forward-looking perspective on the cervical cancer diagnostic tests market. It discusses the anticipated market trends, growth opportunities, and challenges that are likely to shape the market in the coming years. Understanding the future outlook is vital for businesses to plan their long-term strategies and capitalize on emerging market trends.
Conclusion
In conclusion, the cervical cancer diagnostic tests market is poised for significant growth, driven by the increasing incidence of cervical cancer, advancements in diagnostic technologies, and growing awareness about early detection. However, challenges such as high costs and limited accessibility in certain regions need to be addressed. By understanding the market dynamics, capitalizing on opportunities, and developing effective strategies, industry participants can navigate the market landscape successfully and contribute to improved cervical cancer diagnostics worldwide.
What is Cervical Cancer Diagnostic Tests?
Cervical Cancer Diagnostic Tests refer to various medical procedures and screenings used to detect cervical cancer in women. These tests include Pap smears, HPV testing, and colposcopy, which help identify abnormal cells and potential cancerous changes in the cervix.
What are the key players in the Cervical Cancer Diagnostic Tests market?
Key players in the Cervical Cancer Diagnostic Tests market include Roche, Hologic, and Abbott, which are known for their innovative diagnostic solutions and technologies. These companies focus on developing advanced testing methods to improve early detection and treatment outcomes, among others.
What are the main drivers of growth in the Cervical Cancer Diagnostic Tests market?
The growth of the Cervical Cancer Diagnostic Tests market is driven by increasing awareness about cervical cancer prevention, advancements in diagnostic technologies, and rising healthcare expenditure. Additionally, government initiatives promoting regular screenings contribute to market expansion.
What challenges does the Cervical Cancer Diagnostic Tests market face?
The Cervical Cancer Diagnostic Tests market faces challenges such as the high cost of advanced diagnostic technologies and varying access to healthcare services across different regions. Additionally, cultural stigmas and lack of awareness in certain populations can hinder screening participation.
What opportunities exist in the Cervical Cancer Diagnostic Tests market?
Opportunities in the Cervical Cancer Diagnostic Tests market include the development of at-home testing kits and the integration of artificial intelligence in diagnostic processes. These innovations can enhance accessibility and accuracy, potentially increasing screening rates.
What trends are shaping the Cervical Cancer Diagnostic Tests market?
Trends in the Cervical Cancer Diagnostic Tests market include the shift towards personalized medicine and the use of molecular diagnostics. There is also a growing emphasis on preventive healthcare, leading to increased investments in research and development of new testing methodologies.
Cervical Cancer Diagnostic Tests Market:
| Segmentation Details | Description |
|---|---|
| By Test Type | Pap Smear Test, HPV Test, Colposcopy, Biopsy, Others |
| By End User | Hospitals, Diagnostic Laboratories, Clinics, Others |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Cervical Cancer Diagnostic Tests market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at