444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The market for Cerebral Embolic Protection Devices in Transcatheter Aortic Valve Implantation (TAVI) is experiencing significant growth due to the rising prevalence of cardiovascular diseases and the increasing adoption of minimally invasive procedures. Cerebral embolic protection devices play a crucial role in preventing embolic debris from entering the cerebral circulation during TAVI procedures. These devices help reduce the risk of stroke and other neurological complications, thereby improving patient outcomes.
Meaning
Cerebral embolic protection devices are specialized tools used during TAVI procedures to capture and remove embolic debris, such as plaque and thrombus, that may be dislodged during the valve replacement process. These devices act as filters, allowing the blood to pass through while capturing any potentially harmful particles. By preventing the embolic material from reaching the brain, these devices minimize the risk of stroke and neurological damage.
Executive Summary
The market for cerebral embolic protection devices in TAVI is projected to witness substantial growth in the coming years. The increasing geriatric population, coupled with the rising incidence of aortic valve stenosis, is driving the demand for TAVI procedures. As a result, the need for cerebral embolic protection devices is also growing. These devices offer improved safety and reduce the risk of neurological complications during TAVI, making them an integral part of the procedure.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Rising TAVI Volume: Over 300,000 TAVI procedures are performed annually worldwide, with an estimated 15–20% incremental adoption year-over-year in intermediate- and low-risk cohorts.
Stroke Risk Profile: Despite advances, the periprocedural stroke rate after TAVI remains 2–4%; CEPDs can potentially reduce this by up to 40% in selected populations.
Device Evolution: Next-generation filter and deflector designs reduce profile size to 6–9 Fr, enabling single-access workflows and shorter procedural times.
Regulatory Approvals: Sentinel™ holds FDA and CE mark clearance, while other systems are advancing through PMA and CE-IVD pathways, broadening geographic availability.
Economic Impact: With average acute stroke care costs exceeding USD 30,000 per patient, preventing even a small number of events yields substantial cost savings for healthcare systems.
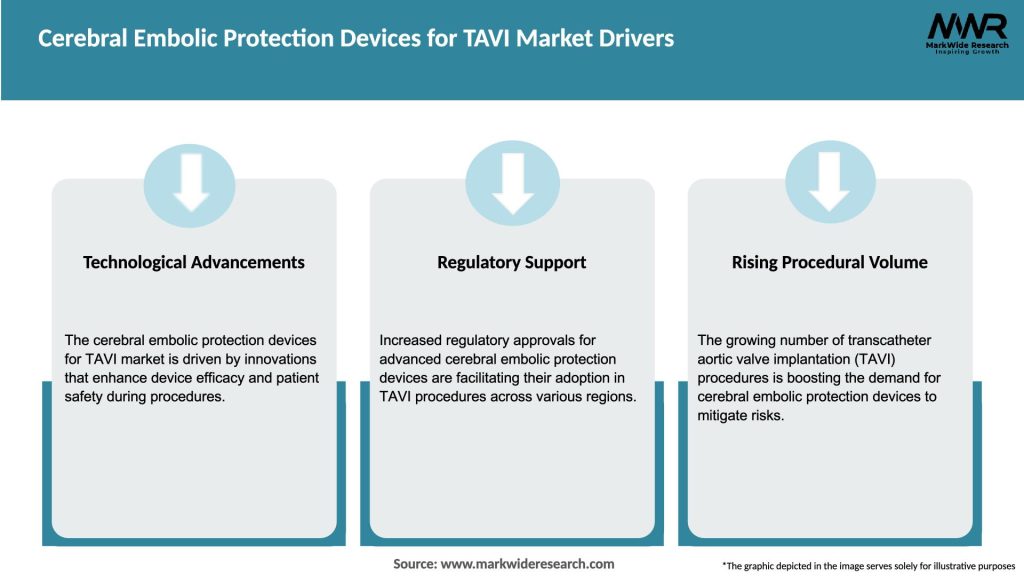
Market Drivers
Clinical Evidence Base: Studies such as the SENTINEL IDE trial and DEFLECT III demonstrated safety, feasibility, and lesion-volume reduction, underpinning clinical confidence.
Guideline Recognition: Emerging commentary in cardiology guidelines advocates considering CEPDs in high-risk TAVI patients, fueling adoption.
Patient Demographics: Expanding TAVI indications to younger, lower-risk patients intensify the focus on minimizing neurologic complications to preserve quality of life.
Reimbursement Policies: Payer coverage for CEPDs is improving as health economic models quantify long-term savings from stroke prevention.
Technological Integration: Improvements in imaging, such as fusion of fluoroscopy and intracardiac echo, streamline CEPD placement and monitoring.
Market Restraints
Added Procedural Complexity: Despite device refinements, CEPD deployment still requires operator training and may prolong procedure duration.
Incremental Costs: Device costs ranging from USD 1,500 to USD 3,000 can be challenging for budget-constrained centers without dedicated reimbursement codes.
Variable Clinical Adoption: Some interventionalists remain unconvinced by mixed trial outcomes, leading to heterogeneous uptake across institutions.
Access Limitations: Tortuous vascular anatomy or severe aortic arch atheroma may preclude safe CEPD placement in a subset of patients.
Data Gaps: Long-term cognitive and functional outcome data are still maturing, tempering universal enthusiasm.
Market Opportunities
Expanded Indications: Investigating CEPD benefits in other transcatheter procedures—such as transcatheter mitral valve repair and left atrial appendage occlusion—broadens market potential.
Integrated TAVI Platforms: Bundling CEPDs with TAVI procedural packages or integrated delivery catheters could simplify workflows and reduce unit costs.
Emerging Economies: Growing TAVI programs in Brazil, India, and China represent significant untapped markets for CEPD adoption as local reimbursement evolves.
Next-Gen Biomaterials: Use of biodegradable or drug-eluting filter materials may enhance safety and further reduce neurological injury.
Digital Health Synergies: Coupling CEPD use with cerebral oximetry and remote neurological monitoring could enable real-time outcome tracking and procedural refinement.

Market Dynamics
Mergers & Partnerships: Collaborations between device OEMs and large hospital systems accelerate guideline development and real-world evidence generation.
Clinical Training Programs: Simulation-based training and proctorship initiatives reduce learning curves and improve CEPD utilization rates.
Value-Based Procurement: Group purchasing organizations and integrated health systems are negotiating bundled TAVI plus CEPD contracts, aligning incentives.
Regulatory Harmonization: Streamlined pathways in Europe, Japan, and the US for post-market data collection facilitate broader device approvals.
Patient Advocacy: Growing patient awareness of stroke risk drives demand for centers that offer CEPDs as part of comprehensive TAVI programs.
Regional Analysis
The market for cerebral embolic protection devices in TAVI is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
Competitive Landscape
Leading Companies in the Cerebral Embolic Protection Devices for TAVI Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The market for cerebral embolic protection devices in TAVI can be segmented based on product type and end-user.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic had a significant impact on the global healthcare industry, including the market for cerebral embolic protection devices in TAVI. During the pandemic, elective procedures, including TAVI, were postponed or canceled due to resource constraints and concerns over patient safety. This led to a temporary decline in the demand for cerebral embolic protection devices.
However, as the situation improved and healthcare facilities resumed normal operations, the market started to recover. The backlog of postponed procedures and the increasing backlog of patients with cardiovascular diseases contributed to the resurgence of the market. Additionally, the focus on minimizing hospital stays and reducing the risk of infection further drove the adoption of minimally invasive procedures like TAVI.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the market for cerebral embolic protection devices in TAVI appears promising. The increasing prevalence of cardiovascular diseases, rising adoption of minimally invasive procedures, and ongoing technological advancements are expected to drive market growth. Furthermore, the untapped opportunities in emerging economies and the focus on product innovation are likely to attract new players and intensify competition. However, challenges such as high costs and the need for skilled professionals remain significant factors to address for sustainable market expansion.
Conclusion
The market for cerebral embolic protection devices in TAVI is experiencing substantial growth due to the increasing demand for minimally invasive procedures and the rising prevalence of cardiovascular diseases. These devices play a crucial role in preventing embolic debris from entering the cerebral circulation, thereby reducing the risk of stroke and neurological complications. While challenges such as high costs and limited awareness exist, opportunities in emerging economies and ongoing product innovation offer promising growth prospects. Companies should focus on research and development, expand market presence, and enhance physician education to capitalize on these opportunities and contribute to improved patient outcomes in TAVI procedures.
What is Cerebral Embolic Protection Devices for TAVI?
Cerebral Embolic Protection Devices for TAVI are specialized medical devices designed to prevent embolic events during transcatheter aortic valve implantation (TAVI) procedures. They work by capturing and removing debris that may dislodge during the procedure, thereby protecting the brain from potential strokes.
What are the key players in the Cerebral Embolic Protection Devices for TAVI Market?
Key players in the Cerebral Embolic Protection Devices for TAVI Market include Abbott Laboratories, Boston Scientific, and Medtronic. These companies are known for their innovative approaches and product offerings in the field of cardiovascular devices, among others.
What are the growth factors driving the Cerebral Embolic Protection Devices for TAVI Market?
The growth of the Cerebral Embolic Protection Devices for TAVI Market is driven by the increasing prevalence of aortic stenosis, advancements in minimally invasive surgical techniques, and a growing aging population. Additionally, the rising awareness of stroke prevention during TAVI procedures contributes to market expansion.
What challenges does the Cerebral Embolic Protection Devices for TAVI Market face?
The Cerebral Embolic Protection Devices for TAVI Market faces challenges such as the high cost of devices, potential complications associated with their use, and varying regulatory approvals across regions. These factors can hinder widespread adoption and market growth.
What opportunities exist in the Cerebral Embolic Protection Devices for TAVI Market?
Opportunities in the Cerebral Embolic Protection Devices for TAVI Market include the development of next-generation devices with improved efficacy and safety profiles, as well as expanding applications in other cardiovascular procedures. Additionally, increasing collaborations between manufacturers and healthcare providers can enhance market reach.
What trends are shaping the Cerebral Embolic Protection Devices for TAVI Market?
Trends shaping the Cerebral Embolic Protection Devices for TAVI Market include the integration of advanced imaging technologies for better device placement and the rise of patient-specific solutions. Furthermore, there is a growing emphasis on clinical studies to validate the effectiveness of these devices in reducing stroke risk.
Cerebral Embolic Protection Devices for TAVI Market
| Segmentation Details | Details |
|---|---|
| Product | Distal Filters, Distal Occlusion Devices |
| End User | Hospitals, Ambulatory Surgical Centers |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Cerebral Embolic Protection Devices for TAVI Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at