444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The Cephalosporin Drugs Market is witnessing robust growth owing to increasing healthcare needs and the prevalence of various infectious diseases. Cephalosporins, a class of antibiotics, have gained prominence due to their effectiveness against a wide range of bacterial infections. This market encompasses a diverse portfolio of cephalosporin drugs, including first, second, third, fourth, and fifth-generation cephalosporins.
Cephalosporin drugs are a class of antibiotics widely used in the treatment of various bacterial infections. They belong to the beta-lactam group of antibiotics and are structurally related to penicillin. Cephalosporins are effective against a broad range of bacteria and are commonly used in both community and hospital settings.
The global cephalosporin drugs market has witnessed significant growth in recent years. This growth can be attributed to the increasing prevalence of bacterial infections, the rise in antibiotic-resistant strains of bacteria, and the growing demand for effective and safe antibiotics. Additionally, advancements in drug development and the introduction of novel cephalosporin drugs have further fueled market growth.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The global cephalosporin drugs market is driven by various factors, including the increasing prevalence of bacterial infections, antibiotic resistance, advancements in drug development, and growing healthcare expenditure. However, there are also restraints such as side effects and allergic reactions associated with cephalosporin drugs, stringent regulatory requirements, and high development and manufacturing costs. Nonetheless, the market presents several opportunities in emerging markets, technological advancements, and the exploration of combination therapies.
Regional Analysis
The cephalosporin drugs market can be analyzed on a regional basis to understand its growth and potential in different geographical areas. The market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa.
North America holds a significant share in the global cephalosporin drugs market due to the presence of well-established healthcare infrastructure, high healthcare expenditure, and a growing burden of bacterial infections. Europe also contributes significantly to the market, driven by increased awareness regarding antibiotic resistance and favorable government initiatives promoting the appropriate use of antibiotics.
The Asia Pacific region is expected to witness substantial growth in the cephalosporin drugs market. Factors such as a large patient population, increasing healthcare investments, and rising awareness about the importance of antibiotic therapy contribute to market growth. Additionally, the presence of major pharmaceutical manufacturing hubs in countries like India and China further supports the market’s expansion.
Latin America and the Middle East and Africa are experiencing steady growth in the cephalosporin drugs market, primarily driven by improvements in healthcare infrastructure, increasing healthcare spending, and the growing prevalence of bacterial infections in these regions.
Competitive Landscape
Leading companies in the Cephalosporin Drugs market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
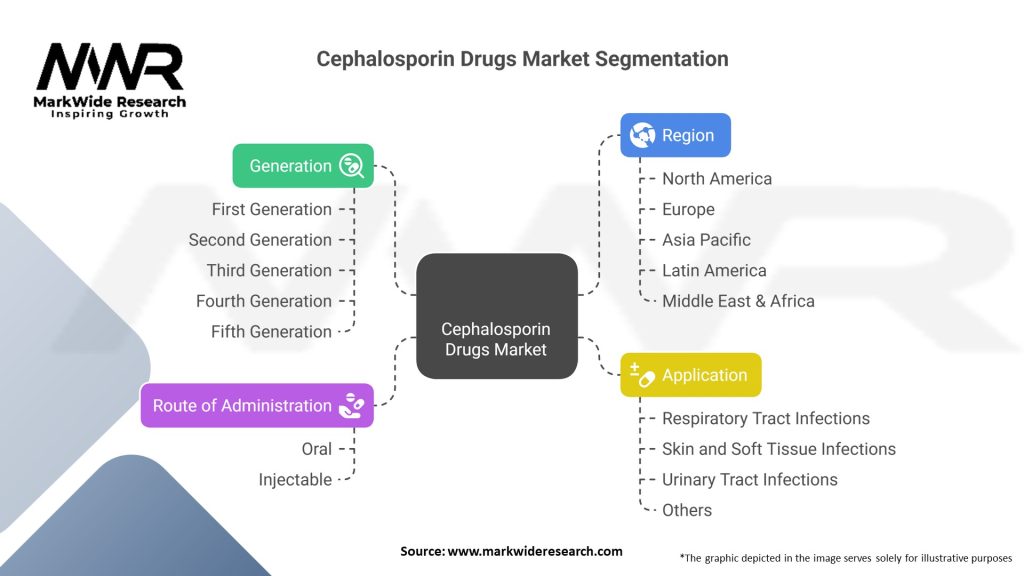
The cephalosporin drugs market can be segmented based on generation, route of administration, and application.
Based on generation, the market is divided into first generation, second generation, third generation, fourth generation, and fifth generation cephalosporin drugs. Each generation exhibits different spectra of activity against bacteria, and their usage depends on the severity and type of infection.
By route of administration, the market can be categorized into oral, parenteral (intravenous or intramuscular), and others. Oral cephalosporin drugs are commonly prescribed for less severe infections, while parenteral administration is preferred for severe infections or when oral intake is not possible.
Regarding application, the market can be segmented into respiratory tract infections, skin and soft tissue infections, urinary tract infections, sexually transmitted diseases, and others. Each application segment represents a specific area of infection where cephalosporin drugs are commonly prescribed.
Category-wise Insights
Within the cephalosporin drugs market, different categories of drugs hold significant importance.
Each category of cephalosporin drugs offers distinct advantages and is tailored to target specific types of bacterial infections. The appropriate selection of the cephalosporin drug category depends on factors such as the type of infection, the susceptibility of the causative bacteria, and the patient’s clinical condition.
Key Benefits for Industry Participants and Stakeholders
Industry participants and stakeholders in the cephalosporin drugs market can derive several benefits from the industry’s growth and evolving landscape:
SWOT Analysis
Strengths:
Established presence of leading pharmaceutical companies with extensive portfolios of cephalosporin drugs across different generations (1st to 5th generation), ensuring strong market positioning.
Robust research and development (R&D) capabilities enabling the development of novel and improved formulations to address evolving bacterial resistance.
Well-developed distribution networks and global market reach ensure efficient supply chain management and availability of drugs across hospitals, clinics, and retail pharmacies.
Strategic collaborations, licensing agreements, and partnerships enhance product development pipelines and support market penetration, particularly in newer therapeutic segments and emerging regions.
Wide application across various bacterial infections, including respiratory, skin, urinary tract, and surgical prophylaxis, supports sustained demand.
Weaknesses:
Side effects and allergic reactions, such as rashes, gastrointestinal disturbances, and in some cases, severe hypersensitivity reactions, can limit patient acceptance and prescribing rates.
Stringent regulatory requirements and complex approval processes for new cephalosporin formulations can delay market entry and increase compliance costs.
High development, manufacturing, and quality assurance costs, especially for newer generation cephalosporins, may impact affordability and restrict accessibility in low-income markets.
The market’s heavy reliance on off-patent drugs and generics limits profitability margins for manufacturers.
Opportunities:
Untapped growth potential in emerging markets like Asia-Pacific, Latin America, and the Middle East due to increasing healthcare infrastructure and rising awareness of antimicrobial therapies.
Technological advancements in drug delivery systems (e.g., extended-release, injectable, and combination formulations) can improve treatment outcomes and patient compliance.
Growing focus on combination therapies involving cephalosporins with β-lactamase inhibitors or other antibiotics to combat resistant bacterial strains.
Increasing demand for broad-spectrum antibiotics in hospital-acquired infection management, surgical prophylaxis, and critical care settings.
Expansion opportunities in veterinary and agricultural applications of cephalosporin drugs for infection control in livestock.
Threats:
Rising concerns of antibiotic resistance pose significant challenges, as cephalosporin overuse and misuse accelerate bacterial resistance, diminishing drug efficacy.
Stringent regulations and antimicrobial stewardship programs aimed at controlling antibiotic use may reduce cephalosporin prescription volumes in certain regions.
Growing competition from alternative treatment options such as novel antibiotic classes, biologics, and phage therapies.
Patent expirations and generic competition leading to price erosion and reduced profitability for branded cephalosporin products.
Public health initiatives promoting reduced antibiotic usage and preventive measures could impact overall demand in the long term.
Market Key Trends
Several key trends are shaping the cephalosporin drugs market:
Covid-19 Impact
The COVID-19 pandemic has had an indirect impact on the cephalosporin drugs market. While the primary focus has been on managing the viral infection, secondary bacterial infections can arise in severe COVID-19 cases. This has led to an increased use of cephalosporin drugs to treat bacterial co-infections.
Additionally, the pandemic has highlighted the importance of infection control measures and appropriate antibiotic use. Efforts to mitigate the spread of antibiotic resistance have gained further significance, with a focus on rational antibiotic prescribing and the development of new drugs to combat emerging resistant strains.
Key Industry Developments
The cephalosporin drugs market has witnessed significant industry developments, including:
Analyst Suggestions
Based on market trends and developments, industry analysts make the following suggestions:
Future Outlook
The future of the cephalosporin drugs market looks promising. Factors such as the increasing prevalence of bacterial infections, the need to combat antibiotic resistance, and ongoing research and development efforts are expected to drive market growth. The introduction of novel cephalosporin drugs, advancements in drug delivery systems, and the exploration of combination therapies will further shape the market.
However, challenges such as stringent regulatory requirements, high development costs, and the emergence of new infectious diseases remain. Overcoming these challenges will require collaborative efforts from industry players, healthcare professionals, and regulatory bodies to ensure the availability of effective and safe cephalosporin drugs.
Conclusion
The cephalosporin drugs market continues to grow as bacterial infections remain a significant healthcare concern globally. The market’s expansion is driven by factors such as the increasing prevalence of bacterial infections, the rise of antibiotic-resistant strains, and advancements in drug development. Additionally, growing healthcare expenditure and favorable government initiatives further contribute to market growth.
While there are challenges such as side effects, stringent regulations, and high costs associated with cephalosporin drugs, opportunities exist in emerging markets, technological advancements, and the development of combination therapies. Collaborations, research and development efforts, and a focus on narrow-spectrum drugs and oral formulations are key trends shaping the market.
Industry participants and stakeholders stand to benefit from revenue generation, market expansion, and contributions to public health. However, it is crucial to maintain regulatory compliance, invest in innovation, and raise awareness about appropriate antibiotic use.
What are cephalosporin drugs?
Cephalosporin drugs are a class of antibiotics used to treat a variety of bacterial infections. They are commonly prescribed for respiratory tract infections, skin infections, and urinary tract infections, among others.
What are the key companies in the Cephalosporin Drugs Market?
Key companies in the Cephalosporin Drugs Market include Pfizer, Merck & Co., GlaxoSmithKline, and Johnson & Johnson, among others.
What are the growth factors driving the Cephalosporin Drugs Market?
The growth of the Cephalosporin Drugs Market is driven by the increasing prevalence of bacterial infections, the rising demand for effective antibiotics, and advancements in pharmaceutical research and development.
What challenges does the Cephalosporin Drugs Market face?
The Cephalosporin Drugs Market faces challenges such as antibiotic resistance, stringent regulatory requirements, and competition from generic drugs, which can impact market growth.
What opportunities exist in the Cephalosporin Drugs Market?
Opportunities in the Cephalosporin Drugs Market include the development of new formulations, expanding into emerging markets, and increasing investment in antibiotic research to combat resistant strains.
What trends are shaping the Cephalosporin Drugs Market?
Trends in the Cephalosporin Drugs Market include the shift towards combination therapies, the focus on personalized medicine, and the growing emphasis on sustainable practices in drug manufacturing.
Cephalosporin Drugs Market
| Segmentation Details | Description |
|---|---|
| Generation | First Generation, Second Generation, Third Generation, Fourth Generation, Fifth Generation |
| Route of Administration | Oral, Injectable |
| Application | Respiratory Tract Infections, Skin and Soft Tissue Infections, Urinary Tract Infections, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Cephalosporin Drugs market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at