444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The cell and gene therapy manufacturing services market represents a rapidly evolving sector within the biotechnology industry, driven by groundbreaking advances in personalized medicine and regenerative therapies. This specialized market encompasses contract development and manufacturing organizations (CDMOs) that provide comprehensive services for the production of cell-based and gene-based therapeutic products. Manufacturing services in this sector include process development, scale-up, clinical manufacturing, and commercial production of advanced therapeutic medicinal products (ATMPs).
Market dynamics indicate robust growth potential, with the sector experiencing a compound annual growth rate (CAGR) of 15.2% as companies increasingly outsource complex manufacturing processes to specialized service providers. The market encompasses various therapeutic modalities including CAR-T cell therapies, gene editing treatments, viral vector-based therapies, and stem cell products. North America currently dominates the market landscape, accounting for approximately 45% of global market share, followed by Europe with 32% market share.
Key market participants include established pharmaceutical companies, emerging biotech firms, and specialized CDMOs that have invested heavily in advanced manufacturing capabilities. The increasing complexity of cell and gene therapies, combined with stringent regulatory requirements, has created significant demand for specialized manufacturing expertise and infrastructure.
The cell and gene therapy manufacturing services market refers to the comprehensive ecosystem of contract manufacturing organizations and service providers that specialize in the development, production, and commercialization of advanced cellular and genetic therapeutic products. These services encompass the entire manufacturing value chain from early-stage process development through large-scale commercial production.
Manufacturing services in this context include cell line development, vector production, cell expansion and differentiation, gene editing, formulation, fill-finish operations, and quality control testing. Service providers offer specialized facilities equipped with cleanroom environments, advanced bioreactors, automated cell processing systems, and sophisticated analytical capabilities required for producing complex biological products.
The market serves pharmaceutical companies, biotechnology firms, academic institutions, and research organizations that require specialized manufacturing capabilities for their cell and gene therapy programs. Contract manufacturing has become increasingly critical as companies seek to access specialized expertise while minimizing capital investment in complex manufacturing infrastructure.
Market expansion in cell and gene therapy manufacturing services is being driven by the increasing number of clinical trials and regulatory approvals for advanced therapeutic products. The sector has witnessed unprecedented growth as more companies recognize the strategic value of partnering with specialized manufacturing service providers rather than developing in-house capabilities.
Key growth drivers include the rising prevalence of genetic disorders, increasing investment in personalized medicine, and growing adoption of CAR-T cell therapies for cancer treatment. The market benefits from supportive regulatory frameworks, with agencies like the FDA and EMA providing clearer guidance for manufacturing standards and quality requirements.
Technological advancements in automation, process analytical technology, and closed-system manufacturing have significantly improved production efficiency and product quality. Service providers are investing heavily in next-generation manufacturing platforms that can accommodate diverse product types and production scales.
Regional distribution shows North America leading with advanced infrastructure and regulatory support, while Asia-Pacific emerges as a high-growth region with projected growth rates exceeding 18% annually. The competitive landscape features both established CDMOs expanding their capabilities and specialized companies focusing exclusively on cell and gene therapy manufacturing.

Market penetration analysis reveals several critical insights shaping the cell and gene therapy manufacturing services landscape:
Primary market drivers propelling growth in cell and gene therapy manufacturing services include the exponential increase in clinical development programs and the complexity of manufacturing requirements that exceed most companies’ internal capabilities.
Regulatory support has emerged as a significant catalyst, with health authorities worldwide implementing expedited approval pathways for breakthrough therapies. The FDA’s Regenerative Medicine Advanced Therapy (RMAT) designation and similar programs in other regions have accelerated development timelines and increased demand for specialized manufacturing services.
Investment influx from venture capital, pharmaceutical companies, and government initiatives has provided substantial funding for cell and gene therapy development. This capital availability enables companies to advance more programs through clinical trials, directly increasing demand for manufacturing services.
Technological breakthroughs in gene editing technologies like CRISPR-Cas9, improved viral vector systems, and advanced cell expansion techniques have expanded the therapeutic potential of cell and gene therapies. These innovations require sophisticated manufacturing capabilities that most companies prefer to access through specialized service providers.
Patient demand for personalized medicine and treatments for previously incurable diseases continues to drive market expansion. The success of approved CAR-T cell therapies and gene therapies has demonstrated clinical efficacy and created market pull for additional therapeutic options.
Manufacturing complexity represents the most significant restraint in the cell and gene therapy manufacturing services market. The intricate nature of biological products requires specialized expertise, advanced equipment, and stringent quality control measures that create barriers to market entry and limit service provider options.
High capital requirements for establishing compliant manufacturing facilities present substantial challenges for new entrants. The cost of building GMP-compliant cleanrooms, acquiring specialized equipment, and maintaining regulatory compliance creates significant financial barriers that limit market expansion.
Regulatory uncertainty in emerging markets and evolving guidelines for novel therapeutic modalities create hesitation among companies considering manufacturing partnerships. The complexity of regulatory requirements across different jurisdictions adds operational challenges and potential delays.
Skilled workforce shortage poses ongoing challenges as the specialized nature of cell and gene therapy manufacturing requires highly trained personnel with expertise in biotechnology, process engineering, and regulatory compliance. The limited availability of qualified professionals constrains capacity expansion and service quality.
Supply chain vulnerabilities for critical raw materials and specialized reagents can disrupt manufacturing operations. The dependence on single-source suppliers for certain components creates risks that can impact production schedules and product availability.
Emerging therapeutic areas present substantial opportunities for manufacturing service providers, particularly in neurological disorders, cardiovascular diseases, and autoimmune conditions where cell and gene therapies show promising potential. The expansion beyond oncology applications opens new market segments with significant growth potential.
Geographic expansion into developing markets offers opportunities for service providers to establish manufacturing capabilities in regions with growing biotechnology sectors. Countries in Asia-Pacific and Latin America are investing heavily in biotechnology infrastructure and represent untapped markets for specialized manufacturing services.
Technology integration opportunities include the development of automated manufacturing platforms, artificial intelligence-driven process optimization, and advanced analytics for quality control. Service providers that successfully implement these technologies can achieve competitive advantages and improved operational efficiency.
Strategic partnerships with pharmaceutical companies, biotechnology firms, and academic institutions create opportunities for long-term collaborations and dedicated manufacturing arrangements. These partnerships can provide stable revenue streams and support capacity expansion investments.
Platform technologies that can accommodate multiple product types and therapeutic modalities represent significant opportunities for service providers to maximize facility utilization and serve diverse client needs efficiently.
Supply and demand dynamics in the cell and gene therapy manufacturing services market reflect a rapidly evolving landscape where demand consistently outpaces available capacity. The increasing number of clinical programs entering late-stage development creates sustained pressure on manufacturing resources.
Competitive dynamics are characterized by both collaboration and competition among service providers. Companies often collaborate on technology development and regulatory initiatives while competing for client contracts and market share. This dynamic environment encourages innovation and service improvement.
Pricing dynamics reflect the specialized nature of services and limited competition in certain therapeutic areas. Service providers can command premium pricing for complex manufacturing processes, though increasing competition is gradually moderating price increases.
Technology evolution continuously reshapes market dynamics as new manufacturing approaches, automation technologies, and quality control methods emerge. Service providers must continuously invest in technology upgrades to remain competitive and meet evolving client requirements.
Regulatory dynamics influence market development through evolving guidelines, approval requirements, and quality standards. Changes in regulatory expectations can create opportunities for compliant service providers while challenging those with outdated capabilities.
Comprehensive market analysis for the cell and gene therapy manufacturing services sector employs a multi-faceted research approach combining primary and secondary research methodologies. The research framework incorporates quantitative analysis of market trends, competitive positioning, and growth projections alongside qualitative insights from industry stakeholders.
Primary research involves extensive interviews with key market participants including CDMO executives, pharmaceutical company decision-makers, regulatory experts, and technology providers. These interviews provide insights into market dynamics, competitive strategies, and future development plans that inform market projections and trend analysis.
Secondary research encompasses analysis of company financial reports, regulatory filings, patent databases, clinical trial registries, and industry publications. This comprehensive data collection provides quantitative foundations for market sizing, competitive analysis, and trend identification.
Market modeling utilizes advanced analytical techniques to project market growth, segment analysis, and regional development patterns. The modeling approach incorporates multiple variables including clinical trial progression, regulatory approval timelines, and capacity expansion plans.
Data validation processes ensure accuracy and reliability through cross-referencing multiple sources, expert review panels, and statistical verification methods. This rigorous approach provides confidence in market projections and strategic recommendations.
North America maintains its position as the dominant region in cell and gene therapy manufacturing services, driven by advanced biotechnology infrastructure, supportive regulatory environment, and significant investment in research and development. The region benefits from established pharmaceutical companies, leading academic institutions, and specialized CDMOs with extensive experience in advanced therapeutic manufacturing.
United States specifically accounts for the largest share of regional market activity, with major manufacturing hubs in Massachusetts, California, and North Carolina. The presence of leading biotechnology companies and favorable regulatory policies through the FDA continue to attract manufacturing investments and capacity expansion projects.
Europe represents the second-largest regional market, with strong growth driven by supportive regulatory frameworks through the European Medicines Agency and significant government investment in biotechnology development. Key markets include Germany, United Kingdom, and Switzerland, each offering specialized manufacturing capabilities and regulatory expertise.
Asia-Pacific emerges as the fastest-growing region with projected annual growth rates exceeding 20% in several countries. China, Japan, and South Korea are investing heavily in biotechnology infrastructure and manufacturing capabilities, creating opportunities for both domestic and international service providers.
Emerging markets in Latin America and Middle East show increasing interest in cell and gene therapy manufacturing, though development remains in early stages. These regions present long-term opportunities as biotechnology sectors mature and regulatory frameworks develop.
Market leadership in cell and gene therapy manufacturing services is distributed among several categories of providers, each offering distinct capabilities and market positioning strategies.
Competitive strategies focus on capacity expansion, technology advancement, geographic diversification, and strategic partnerships. Leading providers are investing heavily in automated manufacturing systems and next-generation production platforms to maintain competitive advantages.
By Service Type:
By Product Type:
By Application:
Clinical Manufacturing Services represent the largest segment by revenue, driven by the increasing number of cell and gene therapy programs entering clinical development. This category benefits from long-term client relationships and predictable revenue streams as programs progress through development phases.
Process Development Services show the highest growth rates as companies seek to optimize manufacturing processes early in development. MarkWide Research analysis indicates this segment is experiencing annual growth rates of 18-22% as more programs enter development pipelines.
Commercial Manufacturing is emerging as a high-value segment with the increasing number of approved cell and gene therapies. This category offers the highest profit margins but requires significant capital investment in manufacturing infrastructure and regulatory compliance.
Gene Therapy Manufacturing demonstrates strong growth potential, particularly for viral vector production services. The complexity of viral vector manufacturing creates barriers to entry and supports premium pricing for specialized service providers.
Cell Therapy Manufacturing benefits from the success of CAR-T cell therapies and expanding applications in regenerative medicine. This segment requires specialized expertise in cell expansion, genetic modification, and cryopreservation technologies.
Pharmaceutical Companies benefit significantly from partnering with specialized manufacturing service providers, gaining access to advanced capabilities without substantial capital investment. These partnerships enable faster time-to-market, reduced development risks, and improved cost efficiency for complex therapeutic products.
Biotechnology Firms can focus resources on core competencies like research and development while leveraging manufacturing expertise from specialized providers. This approach allows smaller companies to advance multiple programs simultaneously without building internal manufacturing capabilities.
Patients ultimately benefit from improved access to innovative therapies as manufacturing partnerships enable more companies to develop and commercialize cell and gene therapies. The specialized expertise of service providers often results in higher quality products and more reliable supply chains.
Investors find attractive opportunities in the manufacturing services sector due to recurring revenue models, high barriers to entry, and growing demand from the expanding cell and gene therapy market. Service providers often demonstrate more predictable financial performance than product development companies.
Healthcare Systems benefit from the improved efficiency and quality of manufacturing services, which can lead to better patient outcomes and more cost-effective treatment options. Specialized manufacturing often results in more consistent product quality and reduced treatment failures.
Strengths:
Weaknesses:
Opportunities:
Threats:
Automation Integration represents a dominant trend as service providers invest in automated manufacturing systems to improve consistency, reduce contamination risks, and increase production efficiency. Advanced robotics and process control systems are becoming standard in new facility designs.
Platform Standardization is emerging as providers develop flexible manufacturing platforms that can accommodate multiple product types and therapeutic modalities. This approach maximizes facility utilization and reduces client costs through shared infrastructure and expertise.
Geographic Diversification continues as service providers establish manufacturing capabilities in multiple regions to serve global markets and reduce supply chain risks. This trend is particularly pronounced in Asia-Pacific markets where biotechnology sectors are rapidly developing.
Digital Transformation is revolutionizing manufacturing operations through implementation of digital twins, predictive analytics, and real-time monitoring systems. These technologies enable better process control, quality assurance, and operational efficiency.
Sustainability Focus is becoming increasingly important as companies implement environmentally responsible manufacturing practices, waste reduction programs, and energy-efficient operations. This trend responds to both regulatory requirements and corporate responsibility initiatives.
Capacity Expansion Projects dominate industry development activity as leading service providers announce major facility investments to meet growing demand. These projects typically involve hundreds of millions in investment and multi-year development timelines.
Strategic Acquisitions are reshaping the competitive landscape as larger CDMOs acquire specialized companies to expand capabilities and geographic reach. These transactions often focus on acquiring specific technologies or therapeutic expertise.
Technology Partnerships between service providers and technology companies are accelerating innovation in manufacturing processes, automation systems, and quality control methods. These collaborations often result in proprietary manufacturing platforms and competitive advantages.
Regulatory Milestone Achievements include facility approvals, manufacturing licenses, and successful regulatory inspections that validate service provider capabilities and enable commercial production for clients.
Client Partnership Announcements highlight long-term manufacturing agreements, dedicated facility arrangements, and strategic collaborations that provide revenue visibility and support capacity expansion investments.
Investment Prioritization should focus on service providers with demonstrated expertise in multiple therapeutic modalities, strong regulatory track records, and clear capacity expansion strategies. Companies with proprietary technologies or platform approaches may offer superior long-term growth potential.
Risk Management considerations include evaluating client concentration, regulatory compliance history, and financial stability of potential partners. MWR recommends diversifying partnerships across multiple service providers to mitigate supply chain risks.
Technology Assessment is critical for identifying service providers with advanced manufacturing capabilities, automation systems, and digital infrastructure that can support complex product requirements and ensure consistent quality.
Geographic Strategy should consider regional manufacturing capabilities, regulatory environments, and market access requirements. Companies may benefit from partnerships with providers offering global manufacturing networks and regulatory expertise.
Long-term Planning should account for evolving manufacturing requirements, emerging therapeutic modalities, and changing regulatory expectations. Flexible partnership arrangements may provide better adaptation to future market developments.
Market trajectory for cell and gene therapy manufacturing services points toward sustained growth driven by expanding therapeutic applications, increasing clinical success rates, and growing commercial adoption of advanced therapies. The sector is expected to maintain robust growth rates as more companies advance programs through development and commercialization.
Technology evolution will continue transforming manufacturing operations through advanced automation, artificial intelligence applications, and next-generation production platforms. These developments will improve manufacturing efficiency, reduce costs, and enable production of increasingly complex therapeutic products.
Regulatory maturation is expected to provide greater clarity and standardization for manufacturing requirements, potentially reducing compliance costs and accelerating approval timelines. Harmonized international standards may facilitate global manufacturing strategies and market access.
Market consolidation may accelerate as larger service providers acquire specialized companies and smaller players struggle to compete with capital requirements and technical complexity. This consolidation could result in a more concentrated market with fewer but larger service providers.
Emerging applications in areas like neurological disorders, cardiovascular diseases, and aging-related conditions represent significant growth opportunities as cell and gene therapy technologies mature and demonstrate clinical efficacy in new therapeutic areas.
The cell and gene therapy manufacturing services market represents one of the most dynamic and rapidly growing sectors in biotechnology, driven by revolutionary advances in personalized medicine and the increasing success of advanced therapeutic products. The market’s evolution from experimental treatments to commercial reality has created unprecedented demand for specialized manufacturing capabilities that exceed most companies’ internal resources.
Strategic partnerships between pharmaceutical companies and specialized service providers have become essential for successful product development and commercialization. The complexity of manufacturing requirements, combined with substantial capital investment needs and regulatory compliance challenges, strongly favors outsourcing arrangements that leverage specialized expertise and infrastructure.
Future market development will be shaped by continued technological innovation, expanding therapeutic applications, and evolving regulatory frameworks that support broader adoption of cell and gene therapies. Service providers that successfully integrate advanced technologies, maintain regulatory compliance, and develop flexible manufacturing platforms will be best positioned to capitalize on sustained market growth and emerging opportunities in this transformative healthcare sector.
What is Cell and Gene Therapy Manufacturing Services?
Cell and Gene Therapy Manufacturing Services refer to the processes and technologies involved in producing therapeutic products derived from cells and genes. This includes the development, production, and quality control of therapies aimed at treating genetic disorders, cancers, and other diseases.
What are the key players in the Cell and Gene Therapy Manufacturing Services Market?
Key players in the Cell and Gene Therapy Manufacturing Services Market include Novartis, Gilead Sciences, and Bristol-Myers Squibb, among others. These companies are involved in various aspects of therapy development and manufacturing, contributing to advancements in the field.
What are the main drivers of growth in the Cell and Gene Therapy Manufacturing Services Market?
The main drivers of growth in the Cell and Gene Therapy Manufacturing Services Market include increasing investments in research and development, a rising prevalence of genetic disorders, and advancements in manufacturing technologies. These factors are fostering innovation and expanding the market.
What challenges does the Cell and Gene Therapy Manufacturing Services Market face?
The Cell and Gene Therapy Manufacturing Services Market faces challenges such as regulatory hurdles, high production costs, and the complexity of manufacturing processes. These issues can hinder the scalability and accessibility of therapies.
What opportunities exist in the Cell and Gene Therapy Manufacturing Services Market?
Opportunities in the Cell and Gene Therapy Manufacturing Services Market include the potential for personalized medicine, advancements in automation and digital technologies, and collaborations between biotech firms and research institutions. These trends are likely to enhance service offerings and efficiency.
What trends are shaping the Cell and Gene Therapy Manufacturing Services Market?
Trends shaping the Cell and Gene Therapy Manufacturing Services Market include the increasing use of artificial intelligence in manufacturing processes, the rise of contract manufacturing organizations, and a focus on sustainable practices. These trends are influencing how therapies are developed and produced.
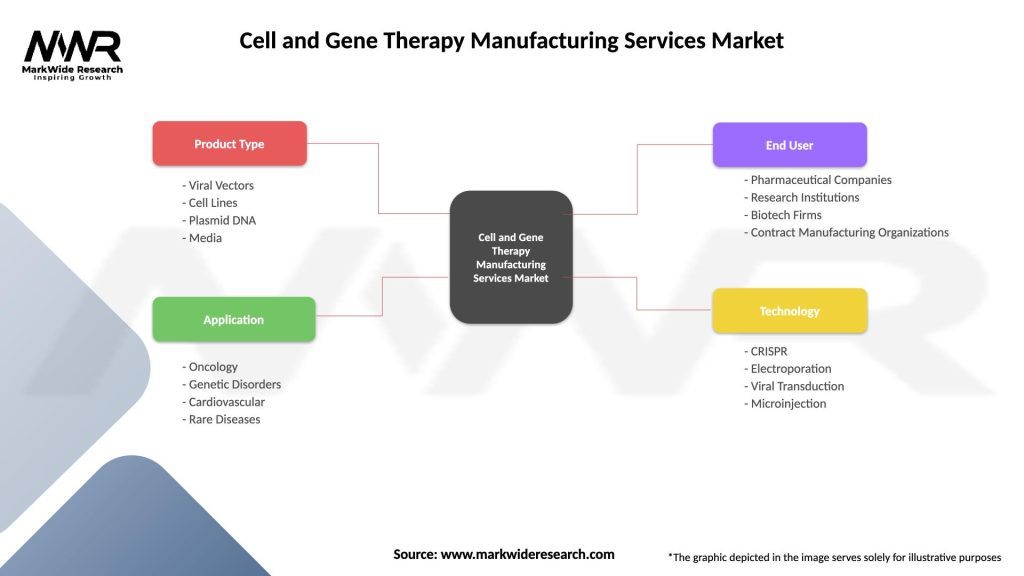
Cell and Gene Therapy Manufacturing Services Market
| Segmentation Details | Description |
|---|---|
| Product Type | Viral Vectors, Cell Lines, Plasmid DNA, Media |
| Application | Oncology, Genetic Disorders, Cardiovascular, Rare Diseases |
| End User | Pharmaceutical Companies, Research Institutions, Biotech Firms, Contract Manufacturing Organizations |
| Technology | CRISPR, Electroporation, Viral Transduction, Microinjection |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Cell and Gene Therapy Manufacturing Services Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at