444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
The Canada thoracolumbar spine devices market represents a critical segment of the country’s orthopedic medical device industry, focusing on innovative solutions for spinal disorders affecting the thoracic and lumbar regions. This specialized market encompasses a comprehensive range of medical devices including spinal fusion systems, vertebral compression fracture devices, motion preservation systems, and minimally invasive surgical instruments designed to treat complex spinal conditions.
Market dynamics in Canada reflect the country’s aging population and increasing prevalence of degenerative spine conditions, driving substantial demand for advanced thoracolumbar spine devices. The market is experiencing robust growth, with adoption rates of minimally invasive procedures increasing by 12% annually across major Canadian healthcare centers. Healthcare infrastructure improvements and technological advancements in spinal surgery techniques are contributing to enhanced patient outcomes and expanding treatment options.
Regional distribution shows concentrated activity in major metropolitan areas including Toronto, Montreal, Vancouver, and Calgary, where specialized spine centers and academic medical institutions drive innovation and adoption of cutting-edge thoracolumbar spine technologies. The market benefits from Canada’s universal healthcare system, which provides structured pathways for device approval and reimbursement, facilitating broader patient access to advanced spinal treatments.
Technology integration is transforming the landscape, with digital surgical planning tools, robotic-assisted procedures, and patient-specific implants gaining significant traction. Canadian spine surgeons are increasingly adopting computer-navigated surgical systems, with utilization rates reaching 38% in major spine centers, reflecting the country’s commitment to precision medicine and improved surgical outcomes.
The Canada thoracolumbar spine devices market refers to the comprehensive ecosystem of medical devices, surgical instruments, and implant systems specifically designed for treating disorders and injuries affecting the thoracic and lumbar regions of the spine within the Canadian healthcare system. This market encompasses both therapeutic and diagnostic solutions that address a wide spectrum of spinal conditions ranging from degenerative disc disease to complex spinal deformities.
Thoracolumbar spine devices include fusion systems utilizing pedicle screws, rods, and interbody cages, as well as motion preservation technologies such as artificial disc replacements and dynamic stabilization systems. The market also covers specialized instruments for minimally invasive procedures, vertebral augmentation devices for compression fractures, and advanced imaging-guided surgical tools that enhance precision and safety during complex spinal interventions.
Market scope extends beyond traditional hardware to include biologics, bone graft substitutes, and emerging technologies like 3D-printed patient-specific implants. The Canadian market is characterized by stringent regulatory oversight through Health Canada, ensuring that all thoracolumbar spine devices meet rigorous safety and efficacy standards before reaching healthcare providers and patients across the country.
Strategic analysis of the Canada thoracolumbar spine devices market reveals a dynamic and rapidly evolving landscape driven by demographic trends, technological innovation, and evolving surgical practices. The market demonstrates strong growth momentum, supported by increasing prevalence of spinal disorders among Canada’s aging population and growing adoption of minimally invasive surgical techniques across the country’s healthcare system.
Key market drivers include the rising incidence of degenerative spine conditions, with lumbar disc degeneration affecting 67% of adults over 50 in Canada, creating substantial demand for therapeutic interventions. The market benefits from robust healthcare infrastructure, skilled spine surgery specialists, and strong research and development capabilities concentrated in major academic medical centers.
Technology trends are reshaping treatment paradigms, with digital surgical planning, robotic assistance, and personalized implant solutions gaining significant adoption. Minimally invasive procedures now account for 45% of all thoracolumbar spine surgeries performed in Canada, reflecting surgeons’ preference for techniques that reduce patient morbidity and accelerate recovery times.
Competitive landscape features both established multinational medical device companies and emerging Canadian innovators developing specialized solutions for the domestic market. The market is supported by favorable reimbursement policies and structured pathways for technology adoption through provincial healthcare systems, creating opportunities for sustained growth and innovation in thoracolumbar spine care.
Market intelligence reveals several critical insights shaping the Canada thoracolumbar spine devices market trajectory. The following key insights provide strategic understanding of market dynamics and growth opportunities:
Primary market drivers propelling growth in the Canada thoracolumbar spine devices market stem from multiple interconnected factors that create sustained demand for advanced spinal treatment solutions. These drivers reflect both demographic realities and technological advancement opportunities within the Canadian healthcare landscape.
Aging population dynamics represent the most significant driver, with Canada’s demographic transition creating unprecedented demand for spine care services. The prevalence of degenerative spine conditions increases dramatically with age, and Canada’s growing elderly population is driving substantial increases in thoracolumbar spine procedures and device utilization across the country.
Technological innovation continues to drive market expansion through the development of more effective, less invasive treatment options. Advanced materials, improved surgical techniques, and digital integration are creating new possibilities for treating complex spinal conditions while reducing patient morbidity and healthcare costs.
Healthcare system evolution supports market growth through improved access to specialized spine care and recognition of the long-term cost benefits of effective spinal interventions. Provincial healthcare systems are increasingly investing in spine care infrastructure and specialist training programs to meet growing demand.
Market challenges facing the Canada thoracolumbar spine devices market include several factors that may limit growth potential or create barriers to market expansion. Understanding these restraints is essential for stakeholders developing strategies to navigate the Canadian healthcare environment effectively.
Cost considerations represent a significant restraint, as advanced thoracolumbar spine devices often require substantial capital investment from healthcare institutions. While Canada’s universal healthcare system provides coverage for medically necessary procedures, budget constraints at provincial levels can impact the adoption rate of newer, more expensive technologies.
Regulatory complexity can slow market entry for innovative devices, as Health Canada’s approval processes, while thorough, require extensive clinical data and documentation. This regulatory pathway, though ensuring safety and efficacy, can delay the availability of breakthrough technologies to Canadian patients and healthcare providers.
Surgeon training requirements for advanced technologies create adoption barriers, as healthcare institutions must invest in comprehensive training programs before implementing new surgical techniques or device systems. This learning curve can slow the uptake of innovative thoracolumbar spine technologies.
Strategic opportunities within the Canada thoracolumbar spine devices market present significant potential for growth and innovation. These opportunities arise from evolving healthcare needs, technological advancement possibilities, and market gaps that innovative companies can address to capture value in this dynamic sector.
Digital health integration offers substantial opportunities for companies developing connected spine devices and digital surgical planning platforms. The integration of artificial intelligence, machine learning, and data analytics into thoracolumbar spine care represents a transformative opportunity for improving patient outcomes and surgical efficiency.
Personalized medicine presents growing opportunities through patient-specific implant design and customized treatment approaches. 3D printing technology adoption for spine implants is accelerating, with custom implant utilization growing 25% annually in major Canadian spine centers, creating opportunities for specialized manufacturing and design services.
Minimally invasive innovation continues to offer expansion opportunities as surgeons seek less traumatic approaches to complex spinal procedures. Companies developing advanced instrumentation and techniques for minimally invasive thoracolumbar spine surgery can capture significant market share in this growing segment.
Market dynamics in the Canada thoracolumbar spine devices sector reflect complex interactions between healthcare policy, technological innovation, demographic trends, and clinical practice evolution. These dynamics create a constantly shifting landscape that requires strategic adaptation from market participants.
Supply chain considerations have gained prominence, particularly following global disruptions that highlighted the importance of reliable device availability. Canadian healthcare institutions are increasingly prioritizing suppliers with robust supply chain management and local manufacturing capabilities to ensure consistent access to critical thoracolumbar spine devices.
Clinical evidence requirements are intensifying as healthcare systems demand stronger proof of device effectiveness and cost-benefit ratios. Real-world evidence collection is becoming essential, with outcome tracking programs now implemented in 78% of major Canadian spine centers, influencing device selection and adoption decisions.
Competitive intensity is increasing as both established players and emerging companies compete for market share in specific device categories. Innovation cycles are accelerating, with companies investing heavily in research and development to differentiate their thoracolumbar spine device offerings in the Canadian market.
Healthcare policy evolution continues to shape market dynamics through changes in reimbursement policies, quality metrics, and patient access programs. Provincial healthcare systems are implementing value-based purchasing approaches that reward devices demonstrating superior patient outcomes and cost-effectiveness.
Comprehensive research methodology employed in analyzing the Canada thoracolumbar spine devices market incorporates multiple data sources and analytical approaches to ensure accuracy and reliability of market insights. The methodology combines quantitative analysis with qualitative research to provide a complete understanding of market dynamics and trends.
Primary research involves extensive interviews with key stakeholders including spine surgeons, hospital administrators, medical device distributors, and healthcare policy experts across Canada. These interviews provide firsthand insights into market trends, adoption patterns, and future outlook for thoracolumbar spine devices in the Canadian healthcare system.
Secondary research encompasses analysis of published clinical studies, healthcare statistics, regulatory filings, and industry reports to establish market baselines and validate primary research findings. MarkWide Research utilizes proprietary databases and analytical tools to process large volumes of market data and identify significant trends and patterns.
Data validation processes ensure research accuracy through triangulation of multiple sources and cross-verification of key findings. The methodology includes statistical analysis of market trends, regression modeling for growth projections, and scenario analysis to account for various market development possibilities.
Regional market analysis reveals significant variations in thoracolumbar spine device adoption and utilization patterns across Canada’s provinces and territories. These regional differences reflect variations in healthcare infrastructure, population demographics, and access to specialized spine care services.
Ontario dominates the Canadian market, accounting for approximately 42% of total thoracolumbar spine device utilization, driven by the province’s large population base and concentration of major academic medical centers in Toronto and Ottawa. The province’s robust healthcare infrastructure and research capabilities make it a key market for innovative spine technologies.
Quebec represents the second-largest regional market, with 23% market share, supported by strong healthcare institutions in Montreal and Quebec City. The province’s unique healthcare system structure and French-language requirements create specific market dynamics that influence device selection and adoption patterns.
Western provinces including British Columbia and Alberta collectively account for 28% of market activity, with Vancouver and Calgary serving as regional spine care centers. These provinces demonstrate strong adoption rates for minimally invasive technologies and robotic surgical systems.
Atlantic provinces and territories represent smaller but growing market segments, with increasing investment in spine care infrastructure and telemedicine capabilities to improve access for patients in remote areas. Regional healthcare networks are collaborating to optimize spine device procurement and utilization across these markets.
Competitive dynamics in the Canada thoracolumbar spine devices market feature a mix of established multinational corporations and innovative specialty companies competing across various device categories and market segments. The competitive environment is characterized by ongoing innovation, strategic partnerships, and focus on clinical outcomes.
Market leaders maintain strong positions through comprehensive product portfolios, established relationships with Canadian healthcare institutions, and robust clinical support programs. These companies invest heavily in surgeon education, clinical research, and regulatory compliance to maintain their competitive advantages.
Competitive strategies focus on clinical differentiation, technological innovation, and value-based partnerships with healthcare institutions. Companies are increasingly emphasizing real-world evidence generation and long-term patient outcome tracking to demonstrate the value of their thoracolumbar spine device solutions.
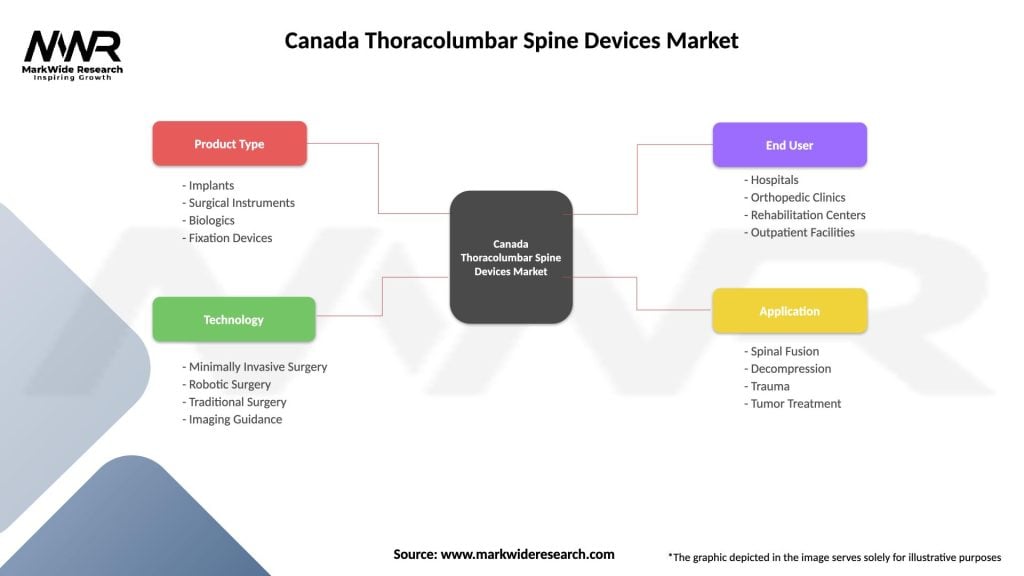
Market segmentation analysis reveals distinct categories within the Canada thoracolumbar spine devices market, each with unique characteristics, growth patterns, and competitive dynamics. Understanding these segments is essential for strategic planning and market positioning.
By Product Type:
By Application:
By End User:
Detailed category analysis provides specific insights into the performance and prospects of major thoracolumbar spine device categories within the Canadian market. Each category demonstrates unique growth patterns and market dynamics.
Spinal Fusion Devices represent the largest category, driven by the high prevalence of degenerative spine conditions requiring stabilization. Posterior fusion procedures account for 58% of all thoracolumbar fusion surgeries in Canada, with pedicle screw systems showing continued innovation in materials and design.
Minimally Invasive Surgery Instruments demonstrate the fastest growth, reflecting surgeon preference for less traumatic approaches. This category benefits from technological advancement in visualization, navigation, and specialized instrumentation designed for smaller incisions and reduced tissue disruption.
Motion Preservation Systems show selective growth in appropriate patient populations, with artificial disc replacement gaining acceptance for specific indications. Canadian spine surgeons are increasingly utilizing motion preservation technologies in younger patients with single-level degenerative conditions.
Vertebral Compression Fracture Devices maintain steady demand driven by osteoporotic fractures in the aging population. Balloon kyphoplasty procedures are particularly popular in Canada, offering effective pain relief and functional improvement for patients with vertebral compression fractures.
Biologics and Bone Graft Substitutes represent a growing category as surgeons seek to enhance fusion rates and reduce complications. Advanced biologics including growth factors and stem cell technologies are gaining adoption in complex revision cases and challenging patient populations.
Strategic benefits for industry participants in the Canada thoracolumbar spine devices market extend across multiple stakeholder groups, creating value through improved patient outcomes, operational efficiency, and market opportunities. Understanding these benefits is essential for effective market participation and stakeholder engagement.
Healthcare Providers benefit from access to advanced thoracolumbar spine technologies that improve surgical outcomes and patient satisfaction. Modern spine devices enable more precise surgical interventions, reduced operative times, and enhanced patient recovery profiles, contributing to improved hospital efficiency and quality metrics.
Patients experience significant benefits through access to innovative treatment options that offer better outcomes with reduced morbidity. Minimally invasive procedures enabled by advanced devices result in shorter hospital stays, faster recovery times, and improved long-term functional outcomes for individuals with thoracolumbar spine conditions.
Medical Device Companies gain access to a stable, well-regulated market with strong healthcare infrastructure and established reimbursement pathways. The Canadian market offers opportunities for sustainable growth, clinical research collaboration, and product development partnerships with leading academic medical centers.
Strengths:
Weaknesses:
Opportunities:
Threats:
Transformative trends are reshaping the Canada thoracolumbar spine devices market, driven by technological innovation, changing clinical practices, and evolving patient expectations. These trends represent fundamental shifts that will define the market’s future direction and competitive landscape.
Minimally Invasive Surgery Adoption continues accelerating, with Canadian spine surgeons increasingly preferring techniques that reduce tissue trauma and improve patient recovery. Percutaneous procedures now represent 34% of all thoracolumbar spine surgeries in major Canadian centers, reflecting this significant trend toward less invasive approaches.
Digital Surgery Integration is transforming surgical planning and execution through advanced imaging, navigation systems, and robotic assistance. Canadian hospitals are investing heavily in digital surgical platforms that enhance precision and improve patient outcomes in thoracolumbar spine procedures.
Personalized Medicine is gaining momentum through patient-specific implant design and customized treatment protocols. 3D printing technology is enabling the creation of tailored spine devices that match individual patient anatomy and biomechanical requirements.
Outcome-Based Healthcare is driving focus on long-term patient results rather than just procedural success. Healthcare systems are implementing comprehensive tracking programs to monitor device performance and patient satisfaction over extended periods.
Recent industry developments in the Canada thoracolumbar spine devices market reflect ongoing innovation, strategic partnerships, and regulatory advancement that are shaping the competitive landscape and market evolution. These developments provide insight into future market direction and opportunities.
Regulatory Advancement includes Health Canada’s implementation of streamlined approval pathways for innovative spine devices, reducing time-to-market for breakthrough technologies while maintaining rigorous safety standards. This regulatory evolution is facilitating faster access to advanced thoracolumbar spine solutions for Canadian patients.
Technology Partnerships between medical device companies and Canadian research institutions are accelerating product development and clinical validation. These collaborations are producing innovative solutions specifically designed for the Canadian healthcare environment and patient population.
Clinical Research Expansion includes major multi-center studies evaluating long-term outcomes of thoracolumbar spine devices in Canadian patient populations. MWR analysis indicates that clinical research investment has increased significantly, supporting evidence-based device selection and adoption.
Infrastructure Investment by provincial healthcare systems includes expansion of spine surgery capacity and implementation of advanced surgical technologies. Major hospitals across Canada are upgrading their spine surgery facilities to accommodate growing demand and technological advancement.
Strategic recommendations for stakeholders in the Canada thoracolumbar spine devices market focus on positioning for sustainable growth while addressing market challenges and capitalizing on emerging opportunities. These analyst suggestions provide actionable guidance for various market participants.
For Medical Device Companies: Focus on developing solutions that demonstrate clear clinical and economic value in the Canadian healthcare context. Invest in local clinical research partnerships and surgeon education programs to build strong relationships with key opinion leaders and healthcare institutions across the country.
For Healthcare Providers: Implement comprehensive spine care programs that integrate advanced technologies with evidence-based clinical protocols. Develop outcome tracking systems to demonstrate value and support continued investment in innovative thoracolumbar spine devices and surgical techniques.
For Investors: Consider the long-term demographic trends and technological advancement opportunities in the Canadian spine device market. Focus on companies with strong clinical evidence, regulatory compliance, and established relationships with Canadian healthcare institutions.
For Policymakers: Continue supporting innovation through balanced regulatory frameworks that ensure safety while facilitating timely access to breakthrough technologies. Consider value-based reimbursement models that reward superior patient outcomes and cost-effectiveness.
Future market prospects for the Canada thoracolumbar spine devices market remain highly positive, supported by favorable demographic trends, technological innovation, and healthcare system evolution. The market is positioned for sustained growth driven by multiple converging factors that create long-term opportunities for stakeholders.
Demographic projections indicate continued aging of the Canadian population, with the number of individuals over 65 expected to increase substantially over the next decade. This demographic shift will drive sustained demand for thoracolumbar spine devices as age-related degenerative conditions become increasingly prevalent.
Technology evolution will continue transforming the market through advancement in minimally invasive techniques, robotic surgery, and personalized medicine. MarkWide Research projects that robotic-assisted spine surgery adoption will reach 25% of procedures in major Canadian centers within the next five years, representing significant growth opportunity.
Healthcare system adaptation will support market growth through improved access to spine care services and recognition of the long-term value of effective spinal interventions. Provincial healthcare systems are expected to continue investing in spine care infrastructure and specialist training programs.
Innovation acceleration in areas such as artificial intelligence, advanced materials, and regenerative medicine will create new treatment possibilities and market opportunities. The integration of digital health technologies with traditional spine devices will enhance patient outcomes and operational efficiency.
Market expansion is expected to continue at a robust pace, with compound annual growth rates projected at 6.8% over the next five years, driven by technology adoption, demographic trends, and healthcare system evolution supporting the thoracolumbar spine devices market in Canada.
The Canada thoracolumbar spine devices market represents a dynamic and rapidly evolving sector within the country’s healthcare landscape, characterized by strong growth fundamentals, technological innovation, and significant opportunities for stakeholders across the value chain. The market benefits from Canada’s robust healthcare infrastructure, aging population demographics, and commitment to advancing spine care through evidence-based medicine and technological adoption.
Market dynamics reflect the complex interplay of demographic trends, technological advancement, regulatory evolution, and healthcare system adaptation that collectively create a favorable environment for sustained growth. The increasing prevalence of degenerative spine conditions, combined with advancing surgical techniques and device technologies, positions the market for continued expansion and innovation.
Strategic opportunities abound for companies that can effectively navigate the Canadian healthcare environment while delivering solutions that demonstrate clear clinical and economic value. The market rewards innovation, clinical excellence, and strong partnerships with healthcare providers and research institutions across the country.
Future success in the Canada thoracolumbar spine devices market will depend on stakeholders’ ability to adapt to evolving healthcare needs, embrace technological innovation, and maintain focus on patient outcomes and value creation. The market’s positive trajectory, supported by demographic trends and technological advancement, creates substantial opportunities for sustained growth and meaningful contribution to improving spine care for Canadian patients.
What is Thoracolumbar Spine Devices?
Thoracolumbar spine devices are medical instruments used to treat conditions affecting the thoracic and lumbar regions of the spine. These devices include implants, braces, and surgical tools designed to stabilize, support, or correct spinal deformities.
What are the key players in the Canada Thoracolumbar Spine Devices Market?
Key players in the Canada Thoracolumbar Spine Devices Market include Medtronic, DePuy Synthes, Stryker, and Zimmer Biomet, among others. These companies are known for their innovative products and extensive research in spinal health.
What are the growth factors driving the Canada Thoracolumbar Spine Devices Market?
The Canada Thoracolumbar Spine Devices Market is driven by an increasing prevalence of spinal disorders, advancements in surgical techniques, and a growing aging population. Additionally, the rise in sports-related injuries contributes to market growth.
What challenges does the Canada Thoracolumbar Spine Devices Market face?
Challenges in the Canada Thoracolumbar Spine Devices Market include high costs of advanced devices, regulatory hurdles, and the need for skilled professionals to perform complex surgeries. These factors can limit accessibility and adoption rates.
What opportunities exist in the Canada Thoracolumbar Spine Devices Market?
Opportunities in the Canada Thoracolumbar Spine Devices Market include the development of minimally invasive surgical techniques and the integration of advanced technologies like robotics and AI. These innovations can enhance patient outcomes and expand market reach.
What trends are shaping the Canada Thoracolumbar Spine Devices Market?
Trends in the Canada Thoracolumbar Spine Devices Market include a shift towards outpatient procedures, increased focus on patient-centered care, and the adoption of biodegradable materials in device manufacturing. These trends aim to improve recovery times and reduce complications.
Canada Thoracolumbar Spine Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Implants, Surgical Instruments, Biologics, Fixation Devices |
| Technology | Minimally Invasive Surgery, Robotic Surgery, Traditional Surgery, Imaging Guidance |
| End User | Hospitals, Orthopedic Clinics, Rehabilitation Centers, Outpatient Facilities |
| Application | Spinal Fusion, Decompression, Trauma, Tumor Treatment |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Canada Thoracolumbar Spine Devices Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at