444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The Brazil Minimally Invasive Surgery Devices Market refers to the sector within the medical industry that focuses on the development, manufacturing, and distribution of medical devices used in minimally invasive surgeries in Brazil. Minimally invasive surgery (MIS) is a technique that allows surgeons to perform various procedures with smaller incisions, resulting in reduced scarring, faster recovery times, and fewer complications compared to traditional open surgeries. The market for minimally invasive surgery devices in Brazil has been witnessing steady growth in recent years, driven by advancements in technology, increasing adoption of MIS procedures, and a growing focus on improving patient outcomes.
Meaning
Minimally invasive surgery devices are specialized instruments and equipment used in various surgical procedures that involve smaller incisions compared to conventional open surgeries. These devices are designed to enable surgeons to access the surgical site using small incisions and perform the procedure using specialized tools, cameras, and imaging systems. By minimizing the size of the incisions, MIS devices help reduce tissue trauma, blood loss, post-operative pain, and recovery time. The market for these devices in Brazil is witnessing significant growth due to the advantages they offer over traditional open surgeries.
Executive Summary
The Brazil Minimally Invasive Surgery Devices Market has experienced substantial growth in recent years. This growth can be attributed to several factors, including technological advancements, increasing demand for minimally invasive procedures, and a favorable regulatory environment. The market offers a wide range of products, including surgical instruments, laparoscopy devices, endoscopy devices, robotic-assisted surgical systems, and imaging systems. The market is highly competitive, with both domestic and international players vying for market share. However, the COVID-19 pandemic has had a temporary impact on the market, leading to delays in elective surgeries and a shift in healthcare priorities. Nevertheless, the market is expected to recover and continue its growth trajectory in the coming years.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
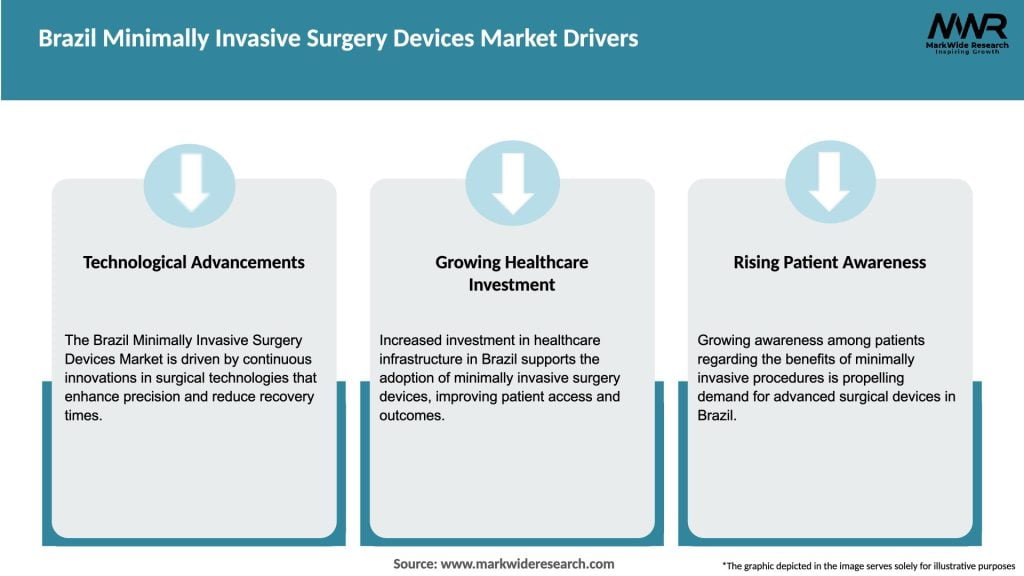
Market Drivers
Several factors are driving the growth of the Brazil Minimally Invasive Surgery Devices market:
Market Restraints
Despite its growth prospects, the Brazil Minimally Invasive Surgery Devices market faces several challenges:
Market Opportunities
The Brazil Minimally Invasive Surgery Devices market presents several opportunities for growth and innovation:

Market Dynamics
The Brazil Minimally Invasive Surgery Devices market is shaped by the following key dynamics:
Regional Analysis
Brazil’s Minimally Invasive Surgery Devices market exhibits regional variations in adoption rates and infrastructure development:
Competitive Landscape
Leading Companies in the Brazil Minimally Invasive Surgery Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Brazil Minimally Invasive Surgery Devices market can be segmented based on various factors:
Category-wise Insights
Each category of minimally invasive surgery devices offers distinct advantages for specific surgical procedures:
Key Benefits for Industry Participants and Stakeholders
The Brazil Minimally Invasive Surgery Devices market offers several benefits for stakeholders:
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Key trends shaping the Brazil Minimally Invasive Surgery Devices market include:
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Brazil Minimally Invasive Surgery Devices Market. The pandemic resulted in a temporary disruption of elective surgeries and a shift in healthcare priorities towards managing the virus. Many hospitals and healthcare facilities had to reallocate resources to handle the surge in COVID-19 cases, leading to delays in non-urgent surgical procedures. Additionally, supply chain disruptions and logistic challenges affected the availability of medical devices, including MIS devices. However, as the situation stabilizes and healthcare systems recover, the market is expected to regain momentum, driven by the pent-up demand for surgical procedures and a focus on improving healthcare infrastructure.
Key Industry Developments
The Brazil Minimally Invasive Surgery Devices Market has witnessed several key industry developments in recent years. These include advancements in robotic-assisted surgical systems, such as the introduction of new robotic platforms with enhanced capabilities. Additionally, there have been developments in laparoscopic instruments, including the integration of advanced energy devices and improved ergonomics. The market has also seen the introduction of novel imaging systems, such as fluorescence imaging, which allows real-time visualization of blood flow and tissue perfusion during surgeries. These industry developments are driving innovation, improving surgical outcomes, and expanding the scope of minimally invasive surgical procedures.
Analyst Suggestions
Based on market analysis and industry trends, analysts suggest several strategies for market participants in the Brazil Minimally Invasive Surgery Devices Market. Firstly, companies should focus on product innovation and the development of advanced devices that address unmet needs in the market. Collaborations with healthcare providers and research institutions can facilitate the identification of key areas for improvement. Secondly, companies should invest in training programs to enhance the skills of healthcare professionals in performing minimally invasive procedures. This can help increase the adoption of MIS techniques and devices. Lastly, companies should stay updated with regulatory changes and ensure compliance with all applicable regulations to avoid delays in product approvals and market entry.
Future Outlook
The future outlook for the Brazil Minimally Invasive Surgery Devices Market is positive, with the market expected to witness steady growth. Factors such as technological advancements, increasing demand for minimally invasive procedures, and a favorable regulatory environment will continue to drive market growth. The market is likely to experience further innovations in robotic-assisted surgical systems, laparoscopic instruments, and imaging technologies. Additionally, efforts to improve healthcare infrastructure in rural areas and expand access to minimally invasive procedures will contribute to market expansion. However, companies must navigate challenges such as high costs, regulatory compliance, and competition to capitalize on the market’s growth potential.
Conclusion
The Brazil Minimally Invasive Surgery Devices Market is witnessing significant growth driven by factors such as technological advancements, increasing demand for minimally invasive procedures, and favorable reimbursement policies. The market offers a wide range of devices, including surgical instruments, laparoscopy devices, endoscopy devices, robotic-assisted surgical systems, and imaging systems. However, challenges such as high costs, regulatory hurdles, and the need for skilled healthcare professionals can impact market growth. To succeed in the market, companies need to focus on innovation, collaboration, and compliance with regulations. Despite the temporary impact of the COVID-19 pandemic, the market is expected to recover and continue its growth trajectory in the coming years.
What is Minimally Invasive Surgery Devices?
Minimally Invasive Surgery Devices refer to medical instruments and technologies that enable surgical procedures with smaller incisions, leading to reduced recovery times and less postoperative pain. These devices are commonly used in various surgical fields, including cardiology, orthopedics, and gynecology.
What are the key players in the Brazil Minimally Invasive Surgery Devices Market?
Key players in the Brazil Minimally Invasive Surgery Devices Market include Medtronic, Johnson & Johnson, Stryker, and Boston Scientific, among others. These companies are known for their innovative products and significant contributions to the development of minimally invasive surgical techniques.
What are the growth factors driving the Brazil Minimally Invasive Surgery Devices Market?
The Brazil Minimally Invasive Surgery Devices Market is driven by factors such as the increasing prevalence of chronic diseases, a growing aging population, and advancements in surgical technologies. Additionally, the rising demand for outpatient surgeries is contributing to market growth.
What challenges does the Brazil Minimally Invasive Surgery Devices Market face?
Challenges in the Brazil Minimally Invasive Surgery Devices Market include high costs associated with advanced surgical technologies and the need for specialized training for healthcare professionals. Furthermore, regulatory hurdles can also impact the speed of product approvals.
What opportunities exist in the Brazil Minimally Invasive Surgery Devices Market?
Opportunities in the Brazil Minimally Invasive Surgery Devices Market include the potential for technological innovations, such as robotic-assisted surgery and enhanced imaging techniques. Additionally, expanding healthcare infrastructure and increasing patient awareness present avenues for growth.
What trends are shaping the Brazil Minimally Invasive Surgery Devices Market?
Trends in the Brazil Minimally Invasive Surgery Devices Market include the rising adoption of robotic surgery systems and the integration of artificial intelligence in surgical procedures. There is also a growing focus on patient-centric approaches and minimally invasive techniques across various surgical specialties.
Brazil Minimally Invasive Surgery Devices Market
| Segmentation Details | Description |
|---|---|
| Product Type | Endoscopes, Trocar Systems, Surgical Staplers, Laparoscopic Instruments |
| Technology | Robotic Surgery, Image-guided Surgery, Laser Surgery, Electrosurgery |
| End User | Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research Institutions |
| Application | Cardiovascular Surgery, Orthopedic Surgery, Gastrointestinal Surgery, Urological Surgery |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Brazil Minimally Invasive Surgery Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at