444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
Atrial Septal Defect (ASD) is a common congenital heart defect that affects the structure of the heart. It occurs when there is a hole in the atrial septum, which is the wall that separates the two upper chambers of the heart. This defect allows blood to flow between the chambers, causing a mixing of oxygenated and deoxygenated blood.
The ASD market refers to the global market for the diagnosis, treatment, and management of atrial septal defects. It includes various medical devices, surgical procedures, and pharmaceutical products aimed at addressing this condition. The market is driven by the increasing prevalence of ASD, advancements in diagnostic techniques and treatment options, and growing awareness among healthcare professionals and patients.
Meaning
An atrial septal defect is a congenital heart defect characterized by a hole in the atrial septum. The atrial septum is the wall that separates the two upper chambers of the heart, namely the left atrium and the right atrium. When there is a hole in this septum, it allows blood to flow abnormally between the chambers, leading to complications in the heart’s function.
ASD can vary in size and location, and the severity of the defect determines the symptoms and potential complications. Small defects may not cause noticeable symptoms, while larger defects can result in symptoms such as fatigue, shortness of breath, and heart palpitations. If left untreated, ASD can lead to serious complications, including heart failure and pulmonary hypertension.
Executive Summary
The market for atrial septal defect (ASD) is expected to experience significant growth in the coming years. This growth can be attributed to several factors, including the rising prevalence of ASD, technological advancements in diagnosis and treatment options, and increasing healthcare expenditure.
The market is witnessing a surge in demand for minimally invasive procedures, such as transcatheter closure, which offer advantages such as shorter recovery times and reduced risk of complications. Additionally, the growing adoption of innovative devices and the development of novel pharmaceutical products contribute to the market’s expansion.
Key players in the market are focusing on research and development activities to introduce advanced technologies and improve treatment outcomes. They are also engaging in strategic collaborations and partnerships to strengthen their market presence and expand their product portfolios.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The atrial septal defect market is driven by a combination of factors, including the prevalence of the condition, advancements in diagnostic techniques, and treatment options. The market dynamics are influenced by various stakeholders, including patients, healthcare professionals, regulatory authorities, and market players.
Patients play a crucial role in driving market growth by seeking early diagnosis and treatment for their condition. Healthcare professionals, including cardiologists and cardiac surgeons, contribute to market dynamics through their expertise in diagnosis, treatment, and follow-up care. Regulatory authorities ensure patient safety and product efficacy by enforcing guidelines and regulations for market approval.
Market players, including medical device manufacturers and pharmaceutical companies, invest in research and development activities to introduce innovative products and gain a competitive edge. These players also collaborate with healthcare institutions and organizations to conduct clinical trials and gather real-world evidence.
Regional Analysis
The atrial septal defect market exhibits regional variations in terms of prevalence, treatment practices, and market dynamics. North America and Europe dominate the market due to the high prevalence of ASD and the presence of well-established healthcare infrastructure. These regions also have favorable reimbursement policies, supporting the adoption of advanced treatment options.
Asia-Pacific is expected to witness significant growth in the atrial septal defect market due to improving healthcare infrastructure, rising disposable incomes, and growing awareness among patients and healthcare professionals. The market in Latin America and the Middle East and Africa is also projected to expand, driven by increasing healthcare expenditure and the introduction of advanced treatment options.
Competitive Landscape
Leading companies in the Atrial Septal Defect Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
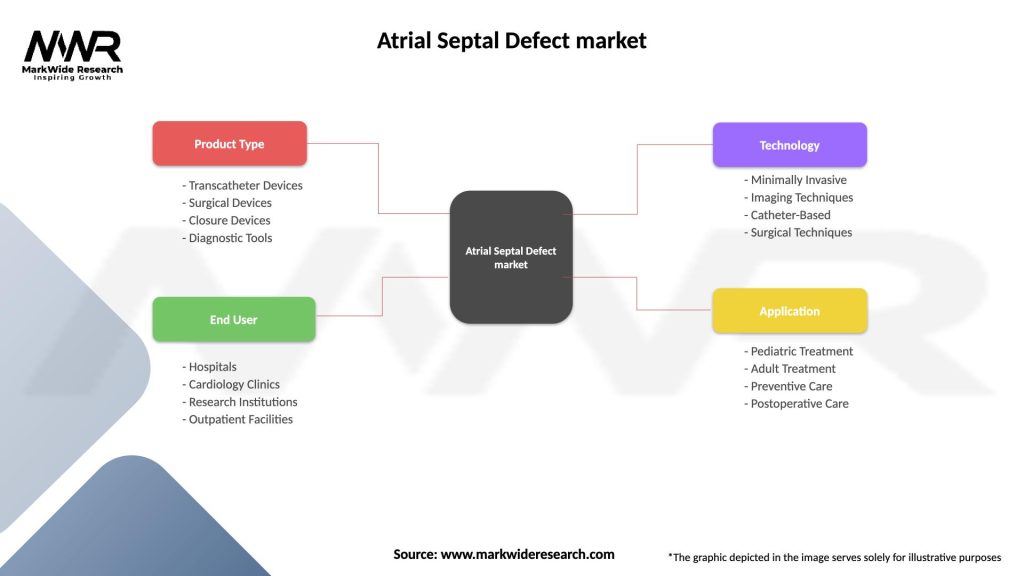
The atrial septal defect market can be segmented based on product type, treatment procedure, end-user, and geography.
By product type:
By treatment procedure:
By end-user:
By geography:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had an impact on the atrial septal defect market. The outbreak resulted in disruptions in healthcare services and a shift in priorities toward managing the pandemic. Elective procedures, including atrial septal defect closures, were postponed or canceled in many regions, leading to a temporary decline in market growth.
However, as healthcare systems adapt to the new normal, the market is expected to recover and witness growth. The emphasis on patient safety and infection control measures has increased, leading to the adoption of minimally invasive procedures such as transcatheter closure, which offer advantages during the pandemic.
The pandemic has also highlighted the importance of remote patient monitoring and telehealth solutions, which can aid in the management of atrial septal defects and ensure continuity of care during times of restricted physical access to healthcare facilities.
Key Industry Developments
Analyst Suggestions
Future Outlook
The atrial septal defect market is expected to grow at a steady pace in the coming years. The increasing prevalence of atrial septal defects, advancements in diagnostic techniques and treatment options, and rising awareness among healthcare professionals and patients are key factors driving market growth.
The market will witness the introduction of innovative medical devices and pharmaceutical products aimed at improving patient outcomes and reducing treatment costs. Technological advancements, such as the integration of artificial intelligence and machine learning, will further enhance diagnosis accuracy and treatment planning.
Emerging markets, particularly in Asia-Pacific and Latin America, offer significant growth opportunities due to improving healthcare infrastructure, rising disposable incomes, and increasing patient awareness. Collaboration between market players and healthcare institutions will continue to drive research and development activities, leading to the introduction of novel treatment options.
Conclusion
The global atrial septal defect market is experiencing significant growth due to the rising prevalence of the condition, advancements in diagnostic techniques and treatment options, and increasing awareness among healthcare professionals and patients. The market offers opportunities for medical device manufacturers, pharmaceutical companies, and healthcare institutions to develop innovative products and improve patient outcomes. Minimally invasive procedures, such as transcatheter closure, are gaining popularity due to their favorable patient outcomes and shorter recovery times. Technological advancements in imaging techniques and the integration of digital health solutions contribute to accurate diagnosis, treatment planning, and patient monitoring.
The atrial septal defect market is poised for growth, driven by the convergence of medical technology advancements, increasing patient awareness, and the commitment of industry participants to improve treatment outcomes and patient care.
What is Atrial Septal Defect?
Atrial Septal Defect (ASD) is a congenital heart defect characterized by an opening in the atrial septum, which separates the heart’s upper chambers. This condition can lead to increased blood flow to the lungs and may result in heart failure if left untreated.
What are the key companies in the Atrial Septal Defect market?
Key companies in the Atrial Septal Defect market include Abbott Laboratories, Medtronic, Boston Scientific, and Edwards Lifesciences, among others.
What are the drivers of growth in the Atrial Septal Defect market?
The growth of the Atrial Septal Defect market is driven by increasing awareness of congenital heart defects, advancements in minimally invasive surgical techniques, and the rising prevalence of heart-related disorders.
What challenges does the Atrial Septal Defect market face?
The Atrial Septal Defect market faces challenges such as high treatment costs, the need for skilled healthcare professionals, and potential complications associated with surgical procedures.
What opportunities exist in the Atrial Septal Defect market?
Opportunities in the Atrial Septal Defect market include the development of innovative closure devices, increasing investment in research and development, and expanding healthcare access in emerging markets.
What trends are shaping the Atrial Septal Defect market?
Trends in the Atrial Septal Defect market include the growing adoption of transcatheter closure techniques, advancements in imaging technologies for diagnosis, and a focus on personalized medicine approaches.
Atrial Septal Defect market
| Segmentation Details | Description |
|---|---|
| Product Type | Transcatheter Devices, Surgical Devices, Closure Devices, Diagnostic Tools |
| End User | Hospitals, Cardiology Clinics, Research Institutions, Outpatient Facilities |
| Technology | Minimally Invasive, Imaging Techniques, Catheter-Based, Surgical Techniques |
| Application | Pediatric Treatment, Adult Treatment, Preventive Care, Postoperative Care |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Atrial Septal Defect Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at