444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The At-Home COVID-19 Test Kits market is a rapidly evolving segment within the healthcare industry, driven by the need for convenient and accessible testing solutions during the COVID-19 pandemic. These test kits enable individuals to self-administer COVID-19 tests at home, providing quick results without the need for laboratory processing. The market for at-home COVID-19 test kits has witnessed exponential growth, fueled by factors such as increasing demand for testing, regulatory approvals for home-use diagnostics, and advancements in testing technology.
Meaning:
At-home COVID-19 test kits are diagnostic tools designed for self-testing of COVID-19 infection outside of traditional healthcare settings. These kits typically include nasal swabs or saliva collection devices, testing reagents, and instructions for sample collection and result interpretation. Users collect their samples at home, perform the test following the provided instructions, and interpret the results within a specified timeframe. At-home test kits offer convenience, privacy, and rapid results, empowering individuals to monitor their health and take appropriate actions to prevent the spread of COVID-19.
Executive Summary:
The At-Home COVID-19 Test Kits market has experienced unprecedented growth in response to the COVID-19 pandemic, with manufacturers ramping up production to meet escalating demand. These test kits play a crucial role in expanding testing capacity, identifying asymptomatic carriers, and containing virus transmission. Regulatory agencies have streamlined approval processes for home-use diagnostics, facilitating market access for test kit manufacturers. Despite challenges such as supply chain disruptions and regulatory hurdles, the At-Home COVID-19 Test Kits market is poised for continued expansion as testing remains a cornerstone of pandemic response efforts.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics
The At-Home COVID-19 Test Kits Market is influenced by several dynamic factors:
Regional Analysis
The At-Home COVID-19 Test Kits Market exhibits regional variations in demand, growth prospects, and market dynamics:
Competitive Landscape
Leading Companies: At-Home COVID-19 Test Kits Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
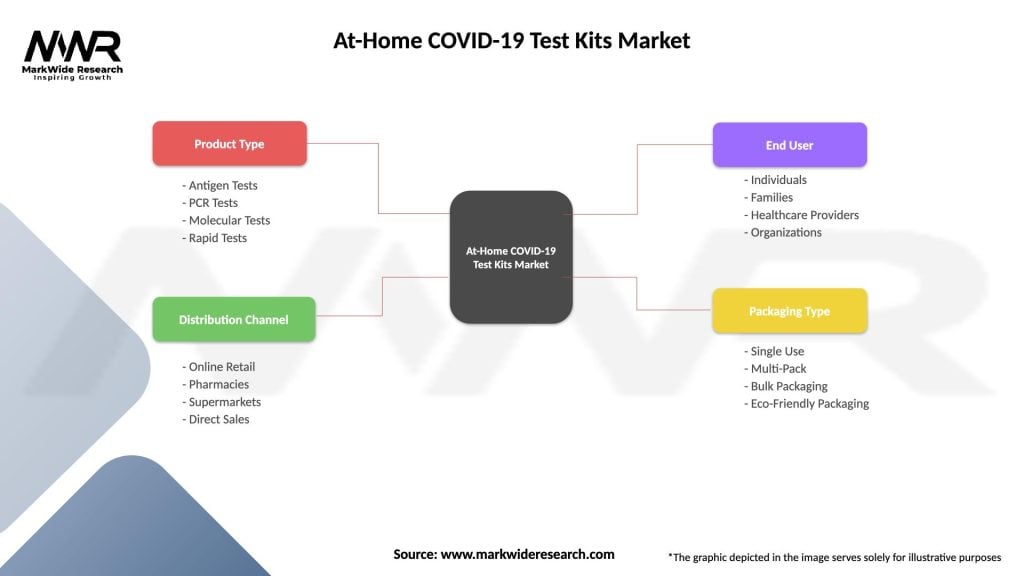
Segmentation
The At-Home COVID-19 Test Kits Market is segmented based on type, distribution channel, and geographic regions:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has significantly impacted the At-Home COVID-19 Test Kits Market:
Key Industry Developments
Analyst Suggestions
Future Outlook
The At-Home COVID-19 Test Kits Market is expected to continue growing, driven by advancements in test technology, increasing consumer demand for convenient solutions, and ongoing regulatory support. Key factors influencing the market’s future include:
Conclusion
The At-Home COVID-19 Test Kits Market is poised for continued growth, driven by advancements in technology, increasing consumer demand, and expanding applications in healthcare. Companies that invest in innovation, address regulatory requirements, and leverage emerging market opportunities will be well-positioned to capitalize on market potential and enhance their competitive edge. The future of the market will be shaped by technological developments, consumer trends, regulatory changes, and economic conditions, providing opportunities for continued growth and innovation in the at-home COVID-19 test kits sector.
What is At-Home COVID-19 Test Kits?
At-Home COVID-19 Test Kits are diagnostic tools that allow individuals to test themselves for COVID-19 from the comfort of their homes. These kits typically include components for sample collection, testing, and result interpretation, making it easier for users to monitor their health without visiting a healthcare facility.
What are the key players in the At-Home COVID-19 Test Kits Market?
Key players in the At-Home COVID-19 Test Kits Market include Abbott Laboratories, Quidel Corporation, and BD (Becton, Dickinson and Company), among others. These companies are known for their innovative testing solutions and have significantly contributed to the accessibility of COVID-19 testing.
What are the main drivers of the At-Home COVID-19 Test Kits Market?
The main drivers of the At-Home COVID-19 Test Kits Market include the increasing demand for convenient testing solutions, the rise in COVID-19 cases, and the growing emphasis on self-monitoring health. Additionally, advancements in testing technology have made these kits more reliable and user-friendly.
What challenges does the At-Home COVID-19 Test Kits Market face?
The At-Home COVID-19 Test Kits Market faces challenges such as regulatory hurdles, concerns about the accuracy of self-administered tests, and potential supply chain disruptions. These factors can impact consumer trust and the overall growth of the market.
What opportunities exist in the At-Home COVID-19 Test Kits Market?
Opportunities in the At-Home COVID-19 Test Kits Market include the potential for expansion into new demographics, the development of more advanced testing technologies, and partnerships with healthcare providers for better distribution. As public awareness of health monitoring increases, the market is likely to grow.
What trends are shaping the At-Home COVID-19 Test Kits Market?
Trends shaping the At-Home COVID-19 Test Kits Market include the integration of digital health technologies, such as mobile apps for result tracking, and the introduction of multi-use test kits that can detect multiple pathogens. These innovations are enhancing user experience and expanding the market’s reach.
At-Home COVID-19 Test Kits Market
| Segmentation Details | Description |
|---|---|
| Product Type | Antigen Tests, PCR Tests, Molecular Tests, Rapid Tests |
| Distribution Channel | Online Retail, Pharmacies, Supermarkets, Direct Sales |
| End User | Individuals, Families, Healthcare Providers, Organizations |
| Packaging Type | Single Use, Multi-Pack, Bulk Packaging, Eco-Friendly Packaging |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies: At-Home COVID-19 Test Kits Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at