444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Asia Pacific Reprocessed Medical Devices market is experiencing significant growth and is expected to witness lucrative opportunities in the coming years. Reprocessed medical devices are those devices that undergo a cleaning, sterilization, and refurbishment process to make them safe for reuse. These devices offer a cost-effective alternative to new medical devices without compromising on quality. The market for reprocessed medical devices in the Asia Pacific region is driven by various factors such as increasing healthcare expenditure, rising awareness about sustainable healthcare practices, and the need to reduce medical waste.
Meaning
Reprocessed medical devices refer to medical equipment that has been used on a patient and then subjected to a process of cleaning, sterilization, and testing to ensure its safety for reuse. These devices can range from surgical instruments to diagnostic equipment and implantable devices. Reprocessing helps healthcare facilities reduce costs associated with purchasing new devices and also contributes to environmentally friendly practices by reducing medical waste.
Executive Summary
The Asia Pacific Reprocessed Medical Devices market is witnessing substantial growth due to the rising demand for cost-effective healthcare solutions. Reprocessed medical devices offer significant cost savings to healthcare providers without compromising patient safety. This has led to increased adoption of reprocessed medical devices in the Asia Pacific region, contributing to market expansion.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
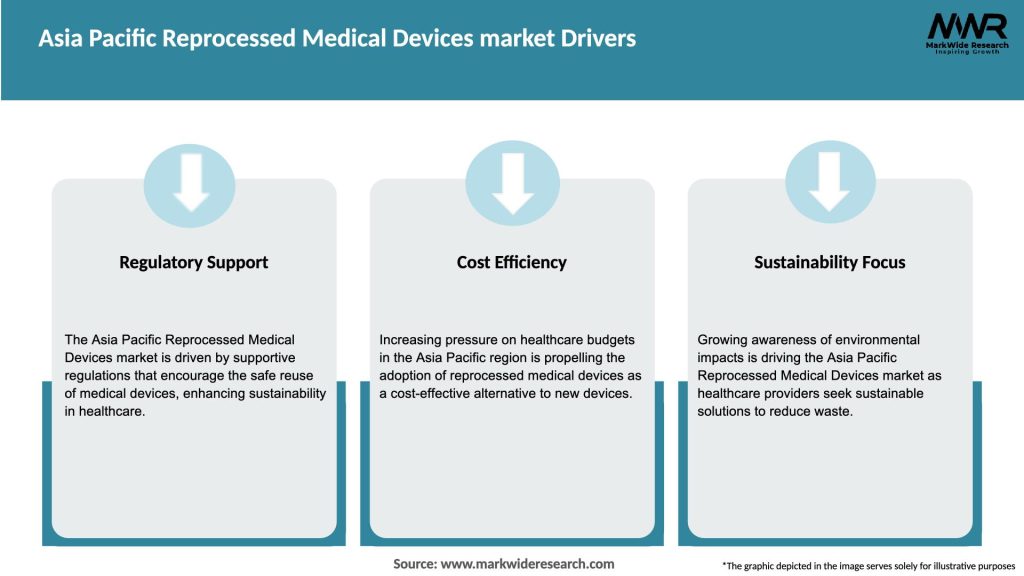
Market Drivers
The Asia Pacific Reprocessed Medical Devices market is driven by several factors:
Market Restraints
Despite the positive growth prospects, the Asia Pacific Reprocessed Medical Devices market faces certain challenges:
Market Opportunities
The Asia Pacific Reprocessed Medical Devices market offers several opportunities for growth and expansion:

Market Dynamics
The Asia Pacific Reprocessed Medical Devices market is driven by dynamic factors such as changing healthcare practices, regulatory landscape, and technological advancements. The market is witnessing a shift towards sustainable and cost-effective healthcare solutions, leading to increased adoption of reprocessed medical devices. However, challenges related to quality control, regulatory compliance, and competition from new devices exist. Opportunities lie in untapped markets, collaborations, technological advancements, and favorable reimbursement policies. Market players need to navigate these dynamics to harness the full potential of the Asia Pacific Reprocessed Medical Devices market.
Regional Analysis
The Asia Pacific Reprocessed Medical Devices market can be analyzed based on regional segments, including:
Competitive Landscape
Leading Companies in the Asia Pacific Reprocessed Medical Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Asia Pacific Reprocessed Medical Devices market can be segmented based on the following factors:
Segmentation allows a deeper understanding of the market, enabling market players to tailor their strategies and offerings to specific segments for better market penetration.
Category-wise Insights
Each category presents unique opportunities and challenges for reprocessing companies, and understanding the specific needs of each category is crucial for market success.
Key Benefits for Industry Participants and Stakeholders
The Asia Pacific Reprocessed Medical Devices market offers several benefits for industry participants and stakeholders:
Understanding these key benefits can help industry participants and stakeholders make informed decisions and leverage the potential of the Asia Pacific Reprocessed Medical Devices market.
SWOT Analysis
A SWOT analysis of the Asia Pacific Reprocessed Medical Devices market provides insights into its internal strengths and weaknesses, as well as external opportunities and threats:
Understanding the SWOT analysis helps market players devise strategies to capitalize on strengths, address weaknesses, seize opportunities, and mitigate threats.
Market Key Trends
The Asia Pacific Reprocessed Medical Devices market is witnessing several key trends:
These key trends shape the Asia Pacific Reprocessed Medical Devices market, indicating the direction it is heading and the opportunities for market players to capitalize on.
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Asia Pacific Reprocessed Medical Devices market:
Overall, the pandemic hasaccelerated the adoption of reprocessed medical devices in the Asia Pacific region as healthcare providers sought cost-effective solutions to meet the increased demand for medical equipment while managing limited resources.
Key Industry Developments
The Asia Pacific Reprocessed Medical Devices market has witnessed several key industry developments:
These industry developments reflect the dynamic nature of the Asia Pacific Reprocessed Medical Devices market and the efforts made by market players to meet the evolving needs of healthcare facilities.
Analyst Suggestions
Based on the analysis of the Asia Pacific Reprocessed Medical Devices market, analysts suggest the following:
Future Outlook
The future outlook for the Asia Pacific Reprocessed Medical Devices market is promising. The market is expected to witness steady growth due to increasing healthcare expenditure, rising awareness about sustainable healthcare practices, and the need to reduce healthcare costs.
The adoption of reprocessed medical devices is likely to increase as healthcare providers recognize the cost-saving benefits and environmental advantages of these devices. Technological advancements in reprocessing techniques will further enhance the safety and efficacy of reprocessed devices, driving their adoption.
Collaborations between healthcare providers, reprocessing companies, and regulatory bodies will play a crucial role in establishing standardized reprocessing practices and ensuring the availability of high-quality reprocessed devices.
However, market players should remain attentive to challenges such as quality control, regulatory compliance, and competition from new devices. Addressing these challenges and capitalizing on emerging opportunities will be key to sustained growth and success in the Asia Pacific Reprocessed Medical Devices market.
Conclusion
The Asia Pacific Reprocessed Medical Devices market is experiencing significant growth and offersnumerous opportunities for market players. Reprocessed medical devices provide cost-effective solutions while promoting sustainable healthcare practices. The market is driven by factors such as increasing healthcare expenditure, rising awareness about environmental conservation, and the need for cost containment.
Despite the positive growth outlook, challenges exist, including limited awareness and skepticism, regulatory requirements, and quality control concerns. However, collaborations, technological advancements, and favorable reimbursement policies present opportunities for market expansion.
The future outlook is positive, with expected growth driven by healthcare expenditure, awareness of sustainable practices, and cost reduction. Continued advancements in reprocessing technologies and collaborations will shape the market. Market players must address challenges and leverage opportunities to thrive in the Asia Pacific Reprocessed Medical Devices market.
What is Reprocessed Medical Devices?
Reprocessed Medical Devices refer to medical instruments that have been cleaned, sterilized, and refurbished for reuse. This practice is often employed to reduce waste and lower healthcare costs while maintaining safety and efficacy standards.
What are the key players in the Asia Pacific Reprocessed Medical Devices market?
Key players in the Asia Pacific Reprocessed Medical Devices market include Stryker Corporation, Medline Industries, and Johnson & Johnson, among others.
What are the growth factors driving the Asia Pacific Reprocessed Medical Devices market?
The growth of the Asia Pacific Reprocessed Medical Devices market is driven by increasing healthcare costs, a rising emphasis on sustainability, and the growing prevalence of chronic diseases that require ongoing medical interventions.
What challenges does the Asia Pacific Reprocessed Medical Devices market face?
Challenges in the Asia Pacific Reprocessed Medical Devices market include regulatory hurdles, concerns regarding the safety and efficacy of reprocessed devices, and competition from new, single-use medical devices.
What opportunities exist in the Asia Pacific Reprocessed Medical Devices market?
Opportunities in the Asia Pacific Reprocessed Medical Devices market include advancements in sterilization technologies, increasing acceptance of reprocessed devices among healthcare providers, and potential partnerships between manufacturers and healthcare facilities.
What trends are shaping the Asia Pacific Reprocessed Medical Devices market?
Trends in the Asia Pacific Reprocessed Medical Devices market include a growing focus on environmental sustainability, innovations in device reprocessing technologies, and an increasing number of hospitals adopting reprocessed devices to reduce costs.
Asia Pacific Reprocessed Medical Devices Market:
| Segmentation Details | Information |
|---|---|
| Device Type | Cardiovascular Devices, Orthopedic Devices, Laparoscopic Devices, Others |
| End User | Hospitals, Ambulatory Surgical Centers, Others |
| Region | Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Malaysia, Thailand, Singapore) |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Asia Pacific Reprocessed Medical Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at