444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Asia-Pacific region is witnessing significant growth in the In-Vitro Diagnostics (IVD) Regulatory Affairs Outsourcing market. As countries in this region continue to focus on improving their healthcare infrastructure, the demand for IVD regulatory affairs services has increased. This market provides assistance to medical device and diagnostic manufacturers in complying with regulatory requirements and obtaining necessary approvals for their products.
Meaning
IVD regulatory affairs outsourcing refers to the practice of engaging external service providers to handle the regulatory affairs processes for IVD products. These services include regulatory strategy development, product registration and submission, quality assurance, compliance testing, and post-market surveillance. By outsourcing these activities to specialized organizations, manufacturers can navigate complex regulatory landscapes and ensure compliance with regional requirements.
Executive Summary
The Asia-Pacific IVD Regulatory Affairs Outsourcing market has witnessed significant growth in recent years. The increasing emphasis on quality healthcare, rising demand for IVD products, and the need for regulatory compliance are the key factors driving this growth. Outsourcing regulatory affairs services allows manufacturers to focus on core competencies while ensuring efficient and timely market entry for their products.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Asia-Pacific IVD Regulatory Affairs Outsourcing market is characterized by intense competition and a dynamic regulatory environment. The market dynamics are influenced by factors such as changing regulatory policies, technological advancements, market consolidation, and collaborations among industry players. Continuous innovation and strategic partnerships are essential to staying competitive in this evolving market.
Regional Analysis
The Asia-Pacific IVD Regulatory Affairs Outsourcing market can be segmented into several key regions, including China, Japan, India, South Korea, Australia, and the Southeast Asian countries. Each region has its own unique regulatory landscape and market dynamics. China and India, in particular, are experiencing robust growth due to favorable government initiatives and increasing healthcare investments.
Competitive Landscape
Leading Companies in the Asia-Pacific IVD Regulatory Affairs Outsourcing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
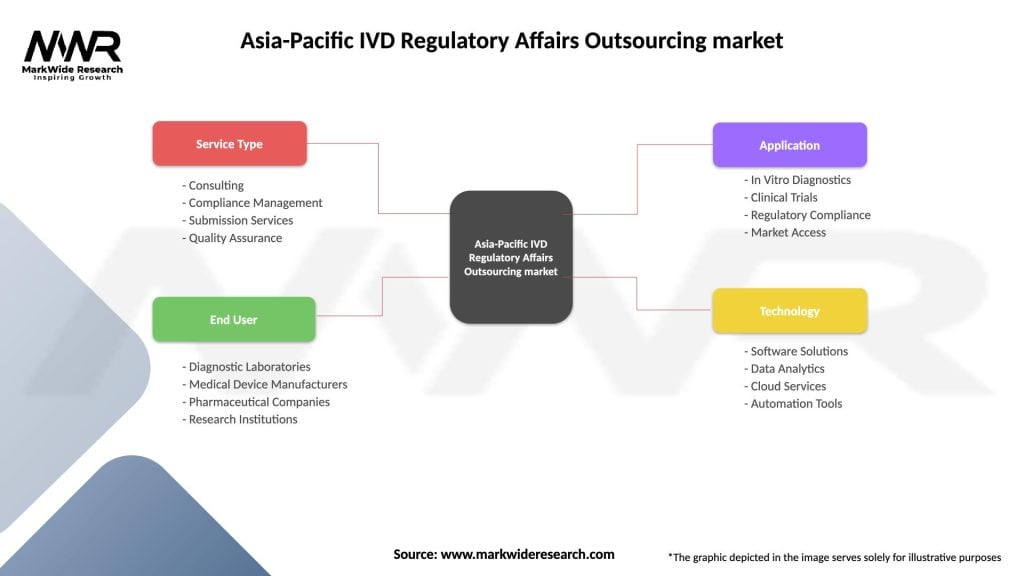
Segmentation
The Asia-Pacific IVD Regulatory Affairs Outsourcing market can be segmented based on the types of services offered, including regulatory strategy development, product registration and submission, quality assurance, compliance testing, and post-market surveillance. The market can also be segmented based on the types of IVD products, such as molecular diagnostics, immunoassays, clinical chemistry, and hematology.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a significant impact on the Asia-Pacific IVD Regulatory Affairs Outsourcing market. The urgent need for diagnostic tests and medical devices to combat the pandemic has led to a surge in demand for regulatory affairs support. Service providers have played a crucial role in facilitating the fast-track approval and market entry of Covid-19 diagnostic tests, ensuring timely access to reliable testing solutions.
The pandemic has also highlighted the importance of regulatory preparedness and agility. Regulatory bodies have implemented emergency use authorization (EUA) pathways and expedited review processes to facilitate the rapid availability of Covid-19 diagnostics. Regulatory affairs service providers have been instrumental in guiding manufacturers through these evolving regulatory frameworks and helping them meet the urgent market demands.
While the immediate focus has been on Covid-19-related products, the pandemic has also underscored the need for robust regulatory systems and effective post-market surveillance. The experience gained during the pandemic is likely to shape future regulatory strategies and strengthen the overall regulatory landscape in the Asia-Pacific region.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Asia-Pacific IVD Regulatory Affairs Outsourcing market is poised for significant growth in the coming years. Factors such as increasing healthcare spending, rising demand for IVD products, and regulatory reforms are expected to drive market expansion. The adoption of advanced technologies, emphasis on post-market compliance, and evolving regulatory harmonization efforts will shape the future of the market.
Manufacturers and regulatory affairs service providers need to adapt to changing market dynamics, leverage digital solutions, and foster collaborations to stay competitive. The industry should anticipate continued regulatory reforms, increased focus on patient safety, and a growing demand for comprehensive regulatory consulting services. The Asia-Pacific region will remain a key market for IVD regulatory affairs outsourcing, presenting lucrative opportunities for industry participants.
Conclusion
The Asia-Pacific IVD Regulatory Affairs Outsourcing market is experiencing robust growth driven by the increasing demand for IVD products, focus on quality healthcare, and the need for regulatory compliance. Outsourcing regulatory affairs processes offers numerous benefits to manufacturers, including accelerated market entry, cost optimization, and access to regional markets.
However, challenges such as data security concerns, lack of harmonization, and communication barriers need to be addressed. The market presents significant opportunities in emerging economies, the adoption of advanced technologies, and collaborations among industry players.
What is IVD Regulatory Affairs Outsourcing?
IVD Regulatory Affairs Outsourcing refers to the practice of delegating regulatory compliance tasks related to in vitro diagnostics to specialized service providers. This includes managing submissions, ensuring adherence to local regulations, and facilitating market access for diagnostic products.
What are the key players in the Asia-Pacific IVD Regulatory Affairs Outsourcing market?
Key players in the Asia-Pacific IVD Regulatory Affairs Outsourcing market include companies like Regulatory Affairs Associates, Emergo, and Qserve, which provide expertise in navigating complex regulatory environments, among others.
What are the main drivers of the Asia-Pacific IVD Regulatory Affairs Outsourcing market?
The main drivers of the Asia-Pacific IVD Regulatory Affairs Outsourcing market include the increasing demand for diagnostic testing, the need for compliance with stringent regulations, and the growing trend of outsourcing to reduce operational costs and enhance efficiency.
What challenges does the Asia-Pacific IVD Regulatory Affairs Outsourcing market face?
Challenges in the Asia-Pacific IVD Regulatory Affairs Outsourcing market include varying regulatory requirements across countries, the complexity of maintaining compliance, and the potential for delays in product approvals due to regulatory scrutiny.
What opportunities exist in the Asia-Pacific IVD Regulatory Affairs Outsourcing market?
Opportunities in the Asia-Pacific IVD Regulatory Affairs Outsourcing market include the expansion of telemedicine and remote diagnostics, advancements in technology that streamline regulatory processes, and the increasing collaboration between regulatory bodies and outsourcing firms.
What trends are shaping the Asia-Pacific IVD Regulatory Affairs Outsourcing market?
Trends shaping the Asia-Pacific IVD Regulatory Affairs Outsourcing market include the rise of digital health solutions, the integration of artificial intelligence in regulatory processes, and a growing emphasis on patient-centric approaches in diagnostics.
Asia-Pacific IVD Regulatory Affairs Outsourcing market
| Segmentation Details | Description |
|---|---|
| Service Type | Consulting, Compliance Management, Submission Services, Quality Assurance |
| End User | Diagnostic Laboratories, Medical Device Manufacturers, Pharmaceutical Companies, Research Institutions |
| Application | In Vitro Diagnostics, Clinical Trials, Regulatory Compliance, Market Access |
| Technology | Software Solutions, Data Analytics, Cloud Services, Automation Tools |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Asia-Pacific IVD Regulatory Affairs Outsourcing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at