444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Asia-Pacific gastrointestinal drugs market refers to the pharmaceutical industry segment that focuses on the development, manufacturing, and distribution of drugs used for the treatment of gastrointestinal disorders. Gastrointestinal disorders encompass a wide range of conditions that affect the digestive system, including but not limited to acid reflux, irritable bowel syndrome, ulcers, inflammatory bowel disease, and liver diseases.
The demand for gastrointestinal drugs in the Asia-Pacific region has been steadily increasing due to various factors, including the rising prevalence of gastrointestinal disorders, growing geriatric population, changing dietary habits, and sedentary lifestyles. This market presents significant opportunities for pharmaceutical companies to develop innovative drugs that effectively address the diverse needs of patients in the region.
Meaning
The Asia-Pacific gastrointestinal drugs market refers to the market segment dedicated to the research, development, production, and distribution of pharmaceutical products used for the treatment of gastrointestinal disorders in the Asia-Pacific region. Gastrointestinal drugs are designed to alleviate symptoms and manage the underlying causes of various digestive system disorders, providing relief to patients and improving their quality of life.
Executive Summary
The Asia-Pacific gastrointestinal drugs market is witnessing substantial growth and is expected to continue expanding in the coming years. Factors such as the rising prevalence of gastrointestinal disorders, increasing healthcare expenditure, and advancements in drug development technologies are driving the market’s growth. Pharmaceutical companies are investing in research and development to introduce innovative drugs that cater to the specific needs of patients in the region. However, challenges such as stringent regulations, pricing pressures, and the impact of the COVID-19 pandemic pose significant hurdles for market players. Despite these challenges, the market presents lucrative opportunities for both established and emerging players.

Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
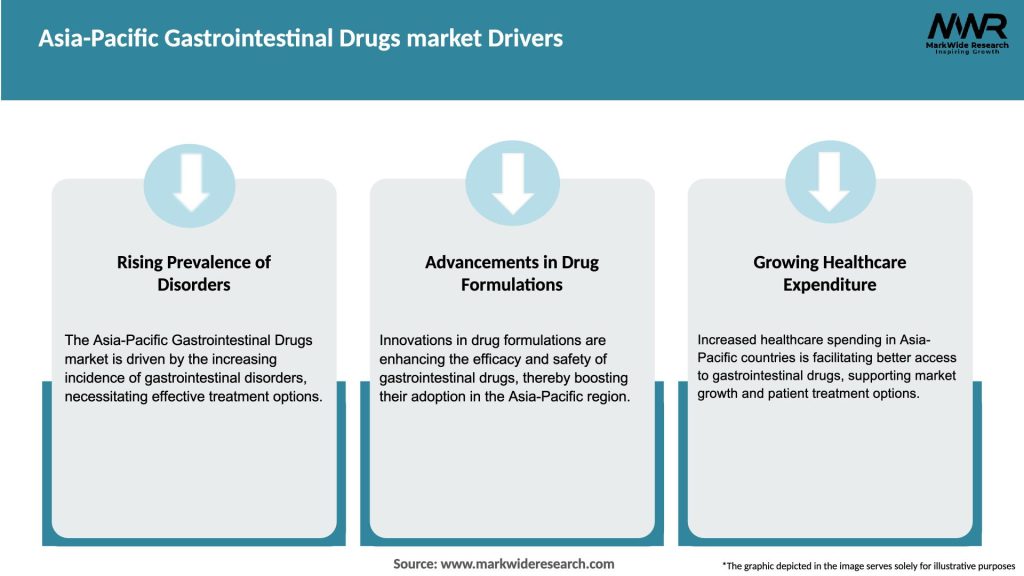
Market Drivers
Several factors are driving the growth of the Asia-Pacific gastrointestinal drugs market:
Market Restraints
Despite the favorable market conditions, the Asia-Pacific gastrointestinal drugs market faces certain challenges that hinder its growth:
Market Opportunities
The Asia-Pacific gastrointestinal drugs market presents several opportunities for pharmaceutical companies and industry participants:
Market Dynamics
The Asia-Pacific gastrointestinal drugs market is influenced by various dynamics that shape its growth and development. These dynamics include market drivers, restraints, opportunities, and trends. Understanding the interplay between these factors is crucial for market players to make informed decisions and capitalize on the emerging opportunities.
The rising prevalence of gastrointestinal disorders, driven by factors such as changing lifestyles and an aging population, acts as a significant driver for market growth. Additionally, advancements in drug development technologies and personalized medicine approaches provide new avenues for innovative treatments.
However, the market also faces challenges, including stringent regulations, pricing pressures, and safety concerns associated with certain drugs. Overcoming these hurdles requires proactive strategies and collaborations to navigate the complex regulatory landscape and develop cost-effective solutions.
Despite these challenges, the market presents numerous opportunities for industry participants. Expanding into untapped markets, investing in research and development, and embracing personalized medicine approaches can unlock significant growth potential. Collaboration and partnerships can also foster innovation and enhance market access.
The market dynamics are further influenced by trends and external factors such as the impact of the COVID-19 pandemic. The pandemic has disrupted supply chains, clinical trials, and healthcare systems, leading to short-term setbacks. However, the demand for gastrointestinal drugs remains resilient, driven by the continued prevalence of gastrointestinal disorders.
Overall, the Asia-Pacific gastrointestinal drugs market is poised for growth, driven by a combination of factors such as disease prevalence, technological advancements, and market opportunities. Industry participants need to adapt to the evolving landscape, leverage emerging trends, and make strategic decisions to capitalize on the market’s potential.
Regional Analysis
The Asia-Pacific gastrointestinal drugs market can be segmented into various regions, including:
China, being the most populous country in the region, holds a significant share of the market. The country’s large patient population, improving healthcare infrastructure, and government initiatives to promote the development of the pharmaceutical sector contribute to its dominance in the market.
Japan is another prominent market in the region, driven by its advanced healthcare system, high healthcare expenditure, andadvanced research and development capabilities. The country has a well-established pharmaceutical industry and is known for its innovation in drug development.
India is emerging as a key player in the Asia-Pacific gastrointestinal drugs market. The country has a large population, which presents a vast patient pool. Additionally, India has a strong generic drug manufacturing sector, making it a hub for affordable medications.
South Korea is witnessing significant growth in the gastrointestinal drugs market. The country’s healthcare infrastructure is highly advanced, and there is a growing demand for innovative treatment options. South Korean pharmaceutical companies are actively engaged in research and development activities, contributing to the market’s expansion.
Australia, with its well-developed healthcare system, offers a favorable environment for the growth of the gastrointestinal drugs market. The country has a high prevalence of gastrointestinal disorders, creating a demand for effective treatment options.
Southeast Asian countries are experiencing rapid economic growth and improvements in healthcare infrastructure. The rising disposable income and increasing healthcare expenditure in these countries contribute to the growth of the gastrointestinal drugs market. These countries also have a high burden of gastrointestinal disorders, driving the demand for medications.
Understanding the regional dynamics, including market size, regulatory landscape, healthcare infrastructure, and disease prevalence, is essential for market players to formulate region-specific strategies and effectively penetrate these markets.
Competitive Landscape
Leading Companies in the Asia-Pacific Gastrointestinal Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
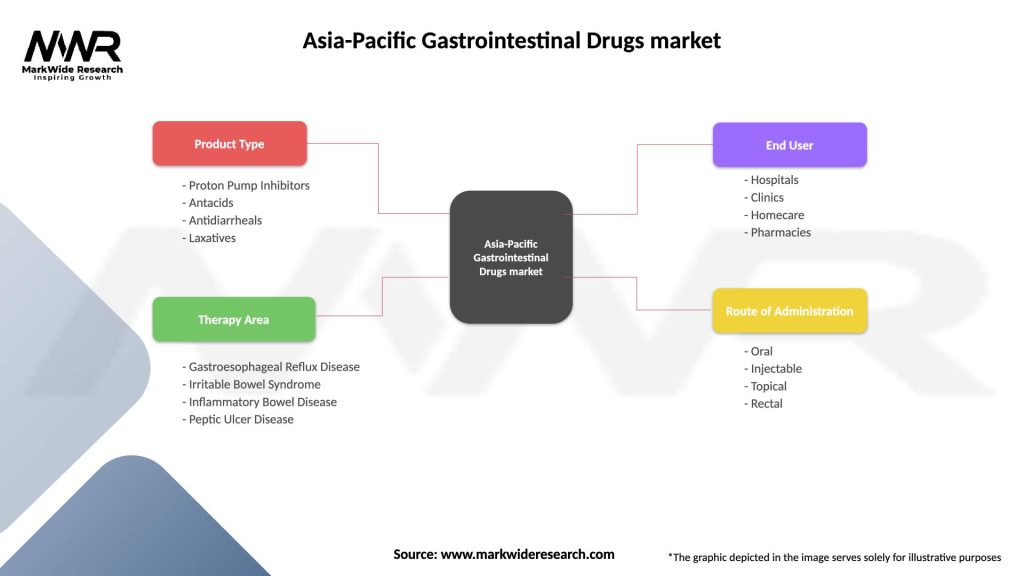
Segmentation
The Asia-Pacific gastrointestinal drugs market can be segmented based on various factors, including:
Segmenting the market allows for a more comprehensive analysis of specific drug categories, disease indications, routes of administration, and distribution channels. This segmentation enables pharmaceutical companies to identify target segments, understand the unique needs of patients, and tailor their marketing and product development strategies accordingly.
Category-wise Insights
Understanding the demand, trends, and specific requirements of each drug category allows pharmaceutical companies to develop targeted strategies and provide optimal solutions to patients in the Asia-Pacific region.
Key Benefits for Industry Participants and Stakeholders
The Asia-Pacific gastrointestinal drugs market offers several key benefits for industry participants and stakeholders:
Understanding these key benefits can guide industry participants and stakeholders in formulating strategies to maximize their engagement in the Asia-Pacific gastrointestinal drugs market.
SWOT Analysis
A SWOT analysis provides an evaluation of the market’s strengths, weaknesses, opportunities, and threats. This analysis helps industry participants gain insights into the internal and external factors that can impact their market performance.
Strengths:
Weaknesses:
Opportunities:
Threats:
By understanding the market’s strengths, weaknesses, opportunities, and threats, industry participants can devise strategies to capitalize on their strengths, address weaknesses, exploit opportunities, and mitigate potential threats.
Market Key Trends
The Asia-Pacific gastrointestinal drugs market is witnessing several key trends that are shaping its growth and development:
Understanding these key trends allows industry participants to stay informed, adapt to evolving market dynamics, and capitalize on emerging opportunities in the Asia-Pacific gastrointestinal drugs market.
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Asia-Pacific gastrointestinal drugs market. The pandemic led to disruptions in the healthcare sector, including supply chains, clinical trials, and patient access to healthcare facilities. However, the demand for gastrointestinal drugs remained strong during this period.
The pandemic’s impact on the market can be summarized as follows:
While the pandemic posed challenges to the Asia-Pacific gastrointestinal drugs market, the demand for these medications remained resilient. As healthcare systems adapt to the new normal, the market is expected to recover and continue its growth trajectory.
Key Industry Developments
The Asia-Pacific gastrointestinal drugs market has witnessed several key industry developments in recent years. These developments have shaped the market landscape and have significant implications for industry participants:
These key industry developments shape the competitive landscape, market dynamics, and treatment options available in the Asia-Pacific gastrointestinal drugs market. Staying informed about these developments is crucial for industry participants to navigate the market effectively.
Analyst Suggestions
Based on the analysis of the Asia-Pacific gastrointestinal drugs market, industry analysts make the following suggestions for market players:
By considering these suggestions, industry participants can navigate the Asia-Pacific gastrointestinal drugs market more effectively, capitalize on emerging opportunities, and overcome challenges to achieve sustainable growth and success.
Future Outlook
The future outlook for the Asia-Pacific gastrointestinal drugs market is optimistic, with several factors shaping its growth:
While the market presents significant opportunities, challenges such as stringent regulations, pricing pressures, and safety concerns will persist. Companies that can navigate these challenges, adapt to emerging trends, and address the evolving needs of patients and healthcare systems will be well-positioned for success in the Asia-Pacific gastrointestinal drugs market.
Conclusion
The Asia-Pacific gastrointestinal drugs market is witnessing significant growth and presents lucrative opportunities for pharmaceutical companies. The rising prevalence of gastrointestinal disorders, increasing geriatric population, and changing lifestyles are driving the market’s expansion. Technological advancements, collaborations, and personalized medicine approaches are transforming the treatment landscape. However, challenges such as stringent regulations, pricing pressures, and safety concerns need to be addressed.
By understanding market dynamics, regional variations, and emerging trends, industry participants can formulate effective strategies to penetrate and expand in the Asia-Pacific gastrointestinal drugs market. Embracing innovation, collaborating with stakeholders, ensuring compliance, and adopting patient-centric approaches are key to success. Continuous monitoring of the market, staying informed about regulatory updates, and investing in research and development activities will help companies stay competitive and meet the evolving needs of patients in the region.
What is Gastrointestinal Drugs?
Gastrointestinal drugs are medications used to treat various disorders of the digestive system, including conditions like acid reflux, irritable bowel syndrome, and inflammatory bowel disease. These drugs can be classified into several categories, such as antacids, proton pump inhibitors, and laxatives.
What are the key players in the Asia-Pacific Gastrointestinal Drugs market?
Key players in the Asia-Pacific Gastrointestinal Drugs market include Takeda Pharmaceutical Company, AstraZeneca, Johnson & Johnson, and Pfizer, among others. These companies are involved in the development and marketing of a wide range of gastrointestinal medications.
What are the growth factors driving the Asia-Pacific Gastrointestinal Drugs market?
The Asia-Pacific Gastrointestinal Drugs market is driven by factors such as the increasing prevalence of gastrointestinal disorders, rising awareness about digestive health, and advancements in drug formulations. Additionally, the growing aging population in the region contributes to the demand for these medications.
What challenges does the Asia-Pacific Gastrointestinal Drugs market face?
The Asia-Pacific Gastrointestinal Drugs market faces challenges such as stringent regulatory requirements, high competition among pharmaceutical companies, and the rising cost of drug development. These factors can hinder the timely introduction of new products into the market.
What opportunities exist in the Asia-Pacific Gastrointestinal Drugs market?
Opportunities in the Asia-Pacific Gastrointestinal Drugs market include the development of novel therapies and personalized medicine approaches. Additionally, increasing investment in research and development by pharmaceutical companies presents avenues for innovation and growth.
What trends are shaping the Asia-Pacific Gastrointestinal Drugs market?
Trends shaping the Asia-Pacific Gastrointestinal Drugs market include the rise of biologics and biosimilars, the growing focus on preventive healthcare, and the integration of digital health technologies. These trends are influencing how gastrointestinal disorders are managed and treated.
Asia-Pacific Gastrointestinal Drugs market
| Segmentation Details | Description |
|---|---|
| Product Type | Proton Pump Inhibitors, Antacids, Antidiarrheals, Laxatives |
| Therapy Area | Gastroesophageal Reflux Disease, Irritable Bowel Syndrome, Inflammatory Bowel Disease, Peptic Ulcer Disease |
| End User | Hospitals, Clinics, Homecare, Pharmacies |
| Route of Administration | Oral, Injectable, Topical, Rectal |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Asia-Pacific Gastrointestinal Drugs Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at