444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
Market Overview
The Asia-Pacific Blood Stream Infection (BSI) Testing Market is a critical segment within the broader healthcare diagnostics industry. Bloodstream infection testing involves the identification of pathogens causing infections in the bloodstream, enabling healthcare professionals to diagnose and treat patients effectively. This market plays a pivotal role in ensuring the timely detection and management of bloodstream infections, contributing to improved patient outcomes.
Meaning
Bloodstream infection testing refers to the diagnostic processes and technologies employed to identify and analyze pathogens present in the bloodstream. These infections can have severe consequences if not promptly diagnosed and treated. Bloodstream infection testing encompasses a range of laboratory techniques, including blood cultures, molecular diagnostics, and other advanced methodologies.
Executive Summary
The Asia-Pacific BSI Testing Market has witnessed significant growth, driven by factors such as the increasing prevalence of bloodstream infections, rising awareness about the importance of early diagnosis, and advancements in diagnostic technologies. The market presents lucrative opportunities for healthcare providers, diagnostic laboratories, and manufacturers of BSI testing products.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Asia-Pacific BSI Testing Market operates in a dynamic landscape influenced by factors such as epidemiological trends, technological advancements, healthcare policies, and the overall economic development of countries in the region. Adapting to these dynamics is crucial for market participants to stay competitive and address the evolving healthcare needs.
Regional Analysis
The performance and growth of the Asia-Pacific BSI Testing Market can vary across countries and regions within the continent. Key regions include:
Competitive Landscape
Leading Companies in Asia-Pacific Blood Stream Infection Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
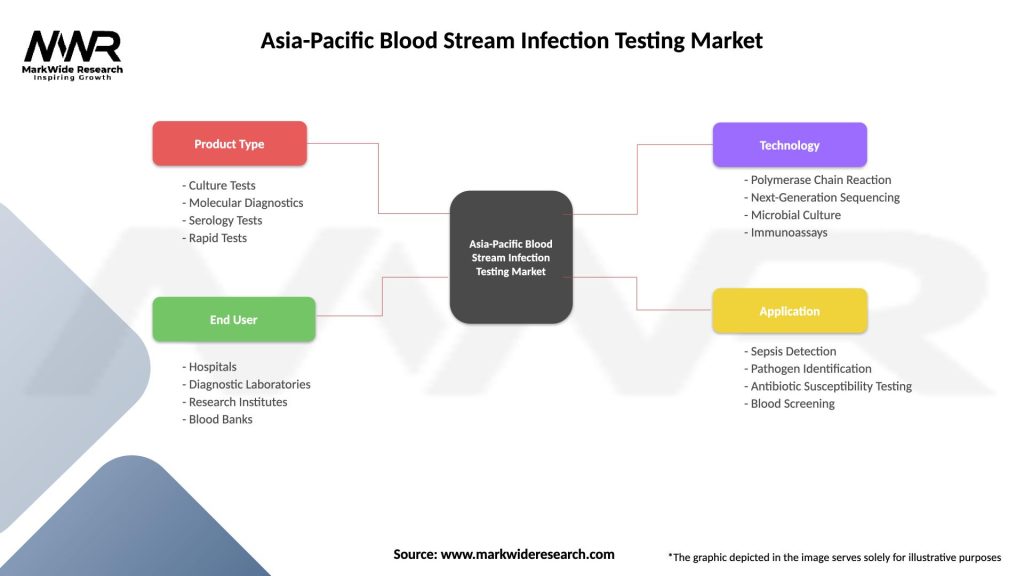
The Asia-Pacific BSI Testing Market can be segmented based on various criteria, including:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT analysis provides an overview of the Asia-Pacific BSI Testing Market’s strengths, weaknesses, opportunities, and threats:
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has influenced the Asia-Pacific BSI Testing Market in several ways:
Key Industry Developments
Analyst Suggestions
Future Outlook
The Asia-Pacific BSI Testing Market is poised for substantial growth in the coming years. The future outlook includes:
Conclusion
The Asia-Pacific Blood Stream Infection Testing Market is a dynamic and evolving sector within the healthcare diagnostics industry. With the rising prevalence of bloodstream infections, increasing awareness about early diagnosis, and ongoing technological advancements, the market presents significant opportunities for industry participants. Overcoming challenges related to awareness, accessibility, and regulatory complexities will be crucial for the sustained growth of the BSI Testing Market in the Asia-Pacific region. As stakeholders collaborate and invest in innovative solutions, the market is poised to play a pivotal role in improving patient outcomes and contributing to the overall healthcare infrastructure of the region.
What is Blood Stream Infection Testing?
Blood Stream Infection Testing refers to the diagnostic processes used to identify pathogens in the bloodstream, which can lead to severe health complications. This testing is crucial for timely treatment and management of infections caused by bacteria, fungi, and other microorganisms.
What are the key players in the Asia-Pacific Blood Stream Infection Testing Market?
Key players in the Asia-Pacific Blood Stream Infection Testing Market include Abbott Laboratories, Becton, Dickinson and Company, and bioMérieux, among others. These companies are known for their innovative diagnostic solutions and technologies in the field of infectious disease testing.
What are the growth factors driving the Asia-Pacific Blood Stream Infection Testing Market?
The growth of the Asia-Pacific Blood Stream Infection Testing Market is driven by increasing incidences of bloodstream infections, advancements in diagnostic technologies, and rising awareness about early detection and treatment. Additionally, the growing geriatric population and the prevalence of chronic diseases contribute to market expansion.
What challenges does the Asia-Pacific Blood Stream Infection Testing Market face?
The Asia-Pacific Blood Stream Infection Testing Market faces challenges such as high costs associated with advanced testing technologies and a lack of skilled professionals to operate sophisticated diagnostic equipment. Furthermore, regulatory hurdles and varying healthcare infrastructure across countries can impede market growth.
What opportunities exist in the Asia-Pacific Blood Stream Infection Testing Market?
Opportunities in the Asia-Pacific Blood Stream Infection Testing Market include the development of rapid testing methods and point-of-care diagnostics. Additionally, increasing investments in healthcare infrastructure and research initiatives aimed at improving infection control measures present significant growth potential.
What trends are shaping the Asia-Pacific Blood Stream Infection Testing Market?
Trends shaping the Asia-Pacific Blood Stream Infection Testing Market include the integration of artificial intelligence in diagnostic processes, the rise of molecular testing techniques, and a focus on personalized medicine. These innovations aim to enhance the accuracy and speed of infection detection.
Asia-Pacific Blood Stream Infection Testing Market
| Segmentation Details | Description |
|---|---|
| Product Type | Culture Tests, Molecular Diagnostics, Serology Tests, Rapid Tests |
| End User | Hospitals, Diagnostic Laboratories, Research Institutes, Blood Banks |
| Technology | Polymerase Chain Reaction, Next-Generation Sequencing, Microbial Culture, Immunoassays |
| Application | Sepsis Detection, Pathogen Identification, Antibiotic Susceptibility Testing, Blood Screening |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Asia-Pacific Blood Stream Infection Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at