444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
Market Overview
The Americas Respiratory Therapeutic Devices market refers to the sector encompassing medical devices and equipment used in the treatment and management of respiratory diseases and disorders in the Americas region. These devices play a crucial role in improving the quality of life for patients suffering from conditions such as asthma, chronic obstructive pulmonary disease (COPD), sleep apnea, and cystic fibrosis. The market for respiratory therapeutic devices in the Americas is driven by the increasing prevalence of respiratory disorders, advancements in technology, and a growing aging population.
Meaning
Respiratory therapeutic devices are specialized medical devices designed to aid in the treatment and management of respiratory conditions. These devices help patients breathe more easily by providing respiratory support, delivering medications, monitoring lung function, and assisting with airway clearance. They can be used in hospitals, clinics, home care settings, and other healthcare facilities. With technological advancements, these devices have become more user-friendly, portable, and efficient, improving patient outcomes and quality of life.
Executive Summary
The Americas Respiratory Therapeutic Devices market has witnessed significant growth in recent years. The market is driven by factors such as the increasing prevalence of respiratory disorders, technological advancements in device design and functionality, and the growing demand for home-based respiratory care solutions. Additionally, favorable reimbursement policies and the rising geriatric population contribute to market growth. However, the market also faces challenges such as pricing pressures, stringent regulatory requirements, and the lack of awareness about respiratory devices among patients and healthcare providers.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
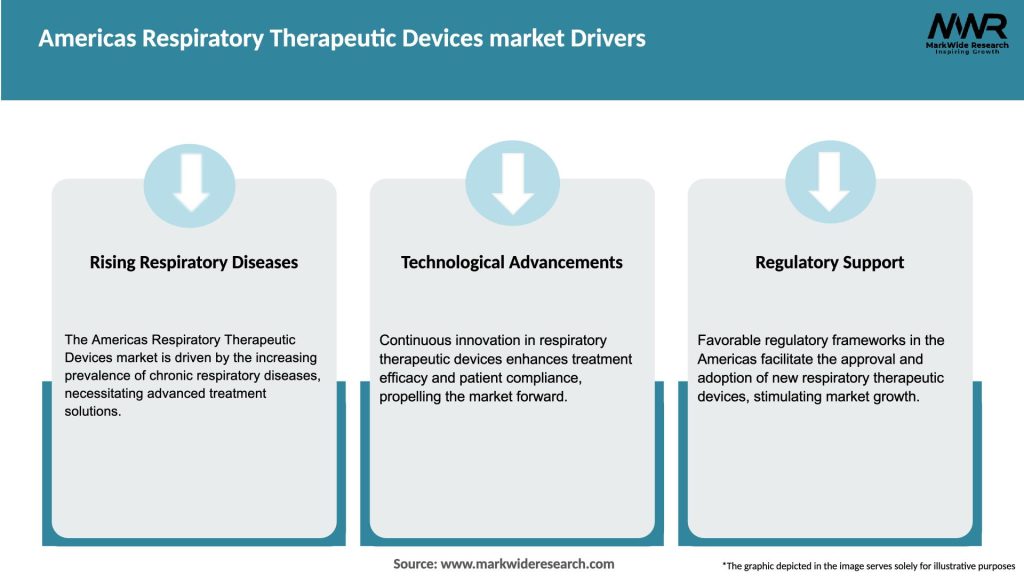
Market Drivers
Market Restraints
Market Opportunities

Market Dynamics
The Americas Respiratory Therapeutic Devices market is driven by several factors, including the increasing prevalence of respiratory disorders, technological advancements, the growing aging population, and favorable reimbursement policies. However, challenges such as pricing pressures, stringent regulatory requirements, lack of awareness, limited access in rural areas, and safety concerns can hinder market growth. Despite these challenges, opportunities exist in the form of home-based care, technological innovations, emerging markets, collaborations, and telehealth integration. Continued focus on research and development, education and awareness programs, and improving access to respiratory therapeutic devices can further drive market growth in the Americas.
Regional Analysis
The Americas Respiratory Therapeutic Devices market can be analyzed based on the regional segmentation of North America, Latin America, and the Caribbean. North America, comprising the United States and Canada, dominates the market due to its advanced healthcare infrastructure, higher healthcare spending, and the presence of key market players. The region has a high prevalence of respiratory disorders, contributing to the demand for respiratory therapeutic devices. Latin America and the Caribbean region show significant growth potential due to the improving healthcare infrastructure, increasing investments in healthcare, and rising awareness about respiratory diseases. These regions present opportunities for market players to expand their market share and cater to the unmet medical needs of the population.
Competitive Landscape
Leading Companies in Americas Respiratory Therapeutic Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Americas Respiratory Therapeutic Devices market can be segmented based on product type, end-user, and geography.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
A SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis of the Americas Respiratory Therapeutic Devices market provides insights into the market’s internal and external factors.
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the Americas Respiratory Therapeutic Devices market. The outbreak led to a surge in respiratory-related illnesses, including severe cases of pneumonia and acute respiratory distress syndrome (ARDS). This resulted in increased demand for respiratory therapeutic devices, particularly ventilators and oxygen concentrators, to support patients with severe respiratory symptoms.
The pandemic also highlighted the importance of telehealth and remote monitoring solutions in managing respiratory conditions. Healthcare providers turned to virtual consultations and remote monitoring to reduce the risk of transmission and ensure continuity of care. This led to the integration of respiratory devices with telehealth platforms, enabling healthcare professionals to remotely monitor patients’ respiratory health and adjust treatment plans accordingly.
However, the pandemic also presented challenges for the respiratory devices market. Supply chain disruptions, increased demand, and the prioritization of resources for COVID-19-related care strained the availability of respiratory devices. Manufacturers faced production constraints, while healthcare facilities experienced shortages of critical respiratory equipment.
Despite the challenges, the pandemic acted as a catalyst for innovation and accelerated the adoption of technology in respiratory care. It emphasized the need for robust respiratory therapeutic devices and highlighted the importance of preparedness in managing respiratory emergencies.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Americas Respiratory Therapeutic Devices market is poised for significant growth in the coming years. The increasing prevalence of respiratory disorders, technological advancements, emphasis on home-based care, and the integration of telehealth solutions will continue to drive market expansion. However, industry participants need to address challenges such as pricing pressures, regulatory compliance, and limited access in rural areas. By focusing on innovation, education, and partnerships, the market can overcome these challenges and improve respiratory care outcomes for patients in the Americas region.
Conclusion
The Americas Respiratory Therapeutic Devices market is witnessing substantial growth driven by factors such as the increasing prevalence of respiratory disorders, technological advancements, and the growing demand for home-based respiratory care solutions. However, challenges such as pricing pressures, regulatory requirements, and limited awareness need to be addressed. Industry participants have opportunities to capitalize on the shift towards personalized care, technological innovations, emerging markets, and collaborations. By focusing on patient-centric solutions, expanding market reach, and continuous research and development, the market can meet the evolving needs of patients and healthcare providers, ultimately improving respiratory health outcomes in the Americas.
What is Respiratory Therapeutic Devices?
Respiratory Therapeutic Devices refer to medical equipment designed to assist patients with respiratory issues, including devices for oxygen therapy, nebulizers, and ventilators. These devices are crucial in managing conditions such as asthma, COPD, and sleep apnea.
What are the key players in the Americas Respiratory Therapeutic Devices market?
Key players in the Americas Respiratory Therapeutic Devices market include Philips Healthcare, ResMed, and Baxter International, among others. These companies are known for their innovative products and significant market presence.
What are the growth factors driving the Americas Respiratory Therapeutic Devices market?
The growth of the Americas Respiratory Therapeutic Devices market is driven by an increasing prevalence of respiratory diseases, a growing aging population, and advancements in technology that enhance device efficiency and patient comfort.
What challenges does the Americas Respiratory Therapeutic Devices market face?
Challenges in the Americas Respiratory Therapeutic Devices market include stringent regulatory requirements, high costs of advanced devices, and competition from alternative therapies. These factors can hinder market growth and innovation.
What opportunities exist in the Americas Respiratory Therapeutic Devices market?
Opportunities in the Americas Respiratory Therapeutic Devices market include the development of portable and home-based devices, increasing telehealth adoption, and the potential for personalized medicine approaches in respiratory care.
What trends are shaping the Americas Respiratory Therapeutic Devices market?
Trends in the Americas Respiratory Therapeutic Devices market include the integration of smart technology in devices, a focus on patient-centered care, and the rise of digital health solutions that enhance monitoring and management of respiratory conditions.
Americas Respiratory Therapeutic Devices market
| Segmentation Details | Description |
|---|---|
| Product Type | Inhalers, Nebulizers, CPAP Devices, Oxygen Concentrators |
| End User | Hospitals, Homecare, Clinics, Long-term Care Facilities |
| Technology | Smart Inhalers, Portable Devices, Digital Monitoring, Non-invasive Ventilation |
| Application | Chronic Obstructive Pulmonary Disease, Asthma, Sleep Apnea, Respiratory Infections |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in Americas Respiratory Therapeutic Devices Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at