444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
The AI in clinical trials market represents a transformative sector within the pharmaceutical and healthcare industries, revolutionizing how medical research is conducted, analyzed, and optimized. This rapidly expanding market encompasses artificial intelligence technologies, machine learning algorithms, and advanced analytics platforms specifically designed to enhance clinical trial efficiency, reduce costs, and accelerate drug development timelines. Market dynamics indicate substantial growth potential driven by increasing demand for precision medicine, regulatory support for digital health technologies, and the urgent need to streamline clinical research processes.
Current market trends demonstrate significant adoption across pharmaceutical companies, biotechnology firms, contract research organizations, and academic medical centers. The integration of AI technologies in clinical trials is experiencing robust growth at a CAGR of 18.2%, reflecting the industry’s commitment to leveraging advanced technologies for improved patient outcomes and operational efficiency. Key market segments include patient recruitment and retention, protocol design optimization, data management and analysis, regulatory compliance, and safety monitoring.
Geographic distribution shows North America maintaining market leadership with approximately 42% market share, followed by Europe at 28% and Asia-Pacific at 22%. The remaining regions collectively represent 8% of the global market presence. Technology adoption rates vary significantly across different clinical trial phases, with Phase II and Phase III trials showing the highest implementation rates of AI-powered solutions.
The AI in clinical trials market refers to the comprehensive ecosystem of artificial intelligence technologies, software platforms, and analytical tools specifically designed to optimize various aspects of clinical research and drug development processes. This market encompasses machine learning algorithms for patient stratification, natural language processing for data extraction, predictive analytics for trial outcome forecasting, and automated systems for regulatory compliance and safety monitoring.
Core components of this market include patient recruitment platforms utilizing AI-driven matching algorithms, protocol optimization tools powered by machine learning, real-world evidence generation systems, and intelligent data management platforms. These technologies collectively aim to reduce clinical trial timelines, improve patient enrollment rates, enhance data quality, and increase the probability of successful drug approvals while maintaining rigorous safety and efficacy standards.
Market transformation in the AI clinical trials sector is being driven by unprecedented technological advancement and industry-wide recognition of artificial intelligence’s potential to address longstanding challenges in medical research. The convergence of big data analytics, machine learning capabilities, and regulatory acceptance has created a favorable environment for widespread adoption of AI-powered clinical trial solutions.
Key growth drivers include the increasing complexity of modern clinical trials, rising development costs requiring efficiency improvements, and the growing emphasis on personalized medicine approaches. Pharmaceutical companies are investing heavily in AI technologies to accelerate drug discovery timelines, with implementation rates increasing by 35% annually across major industry players. Regulatory bodies are also showing increased support for AI integration, with the FDA and EMA providing clearer guidance frameworks for AI-enabled clinical research.
Market challenges include data privacy concerns, regulatory uncertainty in some regions, integration complexity with existing clinical trial management systems, and the need for specialized expertise to implement and maintain AI solutions effectively. Despite these challenges, the market continues to demonstrate strong momentum with significant investment from both private and public sectors.
Strategic market insights reveal several critical trends shaping the AI in clinical trials landscape:
Primary market drivers propelling the AI in clinical trials market include the urgent need for pharmaceutical companies to reduce drug development costs and timelines while maintaining high safety and efficacy standards. Rising development costs in the pharmaceutical industry, which can exceed billions of dollars per approved drug, are creating intense pressure to identify efficiency improvements throughout the clinical research process.
Regulatory support from major health authorities is serving as a significant catalyst for market growth. The FDA’s Digital Health Center of Excellence and similar initiatives from the EMA are providing clearer pathways for AI technology validation and approval in clinical research settings. Data availability has reached unprecedented levels, with electronic health records, wearable devices, and digital biomarkers generating vast amounts of patient information that AI systems can analyze for clinical insights.
Precision medicine initiatives are driving demand for AI technologies capable of identifying patient subgroups most likely to respond to specific treatments. Patient-centric trial designs are becoming increasingly important, with AI enabling more personalized approaches to clinical research that improve patient experience and retention rates. Competitive pressures within the pharmaceutical industry are also accelerating AI adoption as companies seek to gain advantages in drug development speed and success rates.
Significant market restraints include regulatory uncertainty and varying approval processes across different geographic regions, creating complexity for global pharmaceutical companies seeking to implement standardized AI solutions. Data privacy concerns and stringent regulations such as GDPR in Europe and HIPAA in the United States are creating additional compliance burdens for AI system implementations.
Technical challenges related to data integration, system interoperability, and algorithm validation are slowing adoption rates in some organizations. Skilled workforce shortages in AI and data science are limiting the ability of many clinical research organizations to effectively implement and maintain sophisticated AI systems. High implementation costs for comprehensive AI platforms can be prohibitive for smaller biotechnology companies and academic research institutions.
Algorithm bias concerns and the need for diverse, representative datasets are creating additional validation requirements that can delay AI system deployment. Change management challenges within traditional clinical research organizations are also impeding the adoption of AI technologies, as established workflows and processes require significant modification to accommodate new technological approaches.
Emerging market opportunities in the AI clinical trials sector are substantial, particularly in developing markets where traditional clinical research infrastructure may be limited but digital adoption is accelerating rapidly. Decentralized clinical trials represent a significant growth opportunity, with AI technologies enabling remote patient monitoring, virtual consultations, and digital data collection that can expand trial accessibility and reduce geographic barriers.
Real-world evidence generation presents another major opportunity, as AI systems can analyze large-scale patient data from electronic health records, insurance claims, and patient registries to supplement traditional clinical trial data. Rare disease research offers particular promise, as AI can help identify suitable patients across global databases and optimize trial designs for conditions with limited patient populations.
Regulatory technology integration represents a growing opportunity as health authorities seek to modernize their review processes and leverage AI for more efficient drug approvals. Partnership opportunities between technology companies, pharmaceutical firms, and academic institutions are creating new business models and collaborative approaches to AI development in clinical research.
Market dynamics in the AI clinical trials sector are characterized by rapid technological evolution, increasing industry consolidation, and growing collaboration between traditional pharmaceutical companies and technology firms. Competitive pressures are intensifying as companies race to develop and deploy AI solutions that can provide sustainable competitive advantages in drug development efficiency and success rates.
Investment patterns show significant capital flowing into AI clinical trial startups and established technology companies expanding their healthcare portfolios. MarkWide Research analysis indicates that venture capital investment in AI clinical trial technologies has increased substantially, reflecting investor confidence in the sector’s growth potential and transformative impact on pharmaceutical research.
Technology convergence is creating new opportunities as AI systems integrate with other emerging technologies such as blockchain for data integrity, IoT devices for patient monitoring, and cloud computing platforms for scalable data processing. Regulatory evolution continues to shape market dynamics as health authorities develop new frameworks for AI validation and approval in clinical research contexts.
Comprehensive research methodology for analyzing the AI in clinical trials market incorporates multiple data sources and analytical approaches to ensure accuracy and completeness. Primary research includes extensive interviews with pharmaceutical executives, clinical research professionals, technology vendors, and regulatory experts to gather firsthand insights into market trends, challenges, and opportunities.
Secondary research encompasses analysis of published clinical trial data, regulatory filings, patent applications, and academic literature to identify technological developments and market patterns. Market modeling utilizes advanced statistical techniques to project growth trends, segment analysis, and competitive positioning based on historical data and identified market drivers.
Data validation processes include cross-referencing multiple sources, expert review panels, and statistical verification to ensure research findings meet high standards of accuracy and reliability. Continuous monitoring of market developments, regulatory changes, and technological advances ensures that research insights remain current and relevant for strategic decision-making.
North American markets continue to lead global AI clinical trial adoption, driven by strong pharmaceutical industry presence, supportive regulatory environment, and advanced technology infrastructure. The United States represents the largest individual market, with major pharmaceutical companies headquartered in the region investing heavily in AI capabilities and digital transformation initiatives.
European markets demonstrate strong growth potential, particularly in countries with established pharmaceutical industries such as Germany, Switzerland, and the United Kingdom. Regulatory harmonization efforts within the European Union are facilitating cross-border AI clinical trial implementations and creating opportunities for standardized technology platforms.
Asia-Pacific regions are experiencing rapid market expansion, with countries like China, Japan, and India emerging as significant growth markets. Government initiatives supporting digital health and AI development are creating favorable conditions for market growth, while large patient populations provide opportunities for AI algorithm training and validation.
Emerging markets in Latin America, Middle East, and Africa represent future growth opportunities as healthcare infrastructure develops and digital adoption increases. Cost-effective AI solutions designed for resource-constrained environments are beginning to gain traction in these regions.
Market competition in the AI clinical trials sector is intensifying with the participation of established technology companies, specialized AI startups, and traditional clinical research organizations developing AI capabilities. Key market players include:
Competitive strategies focus on developing comprehensive AI platforms, establishing strategic partnerships with pharmaceutical companies, and expanding geographic presence through acquisitions and joint ventures. Innovation leadership is becoming increasingly important as companies compete to develop breakthrough AI technologies that can significantly improve clinical trial outcomes.
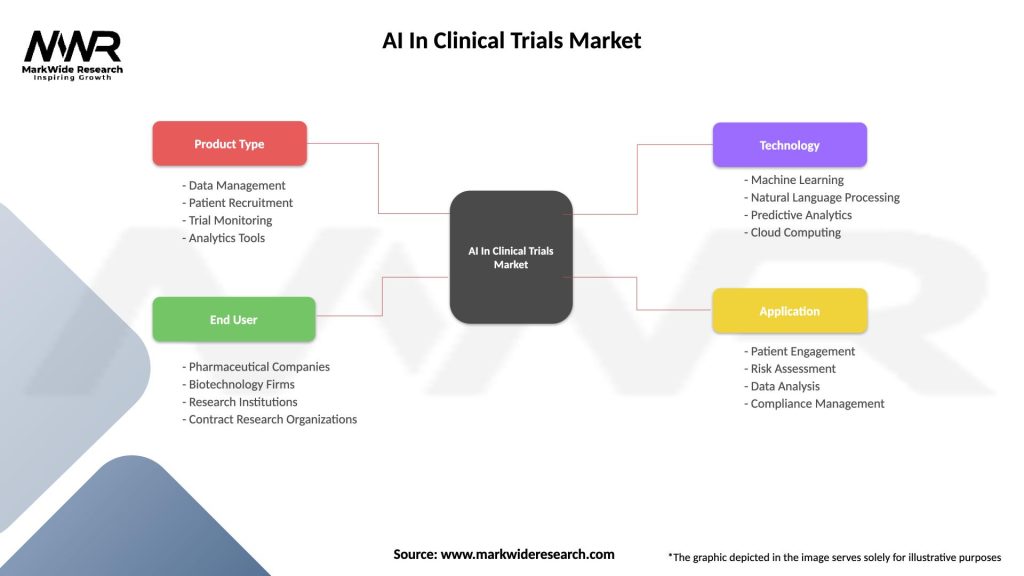
Market segmentation analysis reveals distinct categories based on technology type, application area, end-user, and deployment model. By Technology:
By Application:
By End-User:
Patient recruitment technologies represent the largest and fastest-growing segment within the AI clinical trials market, addressing one of the most significant challenges in clinical research. Advanced algorithms can analyze electronic health records, patient registries, and real-world data sources to identify potential trial participants with unprecedented speed and accuracy.
Protocol optimization tools are gaining significant traction as pharmaceutical companies seek to reduce protocol amendments and improve trial design efficiency. Machine learning models can analyze historical trial data to predict optimal endpoints, patient populations, and study designs that maximize the probability of successful outcomes.
Data management platforms incorporating AI capabilities are transforming how clinical trial data is collected, validated, and analyzed. Automated systems can identify data inconsistencies, predict missing data patterns, and optimize data collection workflows to improve overall data quality and reduce manual oversight requirements.
Safety monitoring applications leverage AI to enhance pharmacovigilance activities and accelerate the detection of potential safety signals. Real-time analysis of adverse event reports, laboratory data, and patient-reported outcomes enables faster identification of safety concerns and more informed risk-benefit assessments.
Pharmaceutical companies benefit from AI clinical trial technologies through reduced development timelines, improved success rates, and enhanced competitive positioning in drug development. Cost optimization achieved through AI implementation can free up resources for additional research and development initiatives, while improved patient recruitment and retention rates enhance trial feasibility and outcomes.
Clinical research organizations gain operational efficiency improvements, enhanced service offerings, and competitive differentiation through AI technology adoption. Automated processes reduce manual workload, minimize errors, and enable staff to focus on higher-value activities that require human expertise and judgment.
Patients and healthcare providers benefit from more efficient trial processes, improved matching to appropriate studies, and enhanced safety monitoring capabilities. Personalized approaches enabled by AI can improve patient experience and outcomes while reducing the burden of clinical trial participation.
Regulatory agencies benefit from improved data quality, enhanced safety monitoring, and more efficient review processes enabled by AI technologies. Standardized approaches to data collection and analysis can facilitate regulatory decision-making and improve the overall quality of clinical evidence.
Strengths:
Weaknesses:
Opportunities:
Threats:
Decentralized clinical trials are emerging as a dominant trend, with AI technologies enabling remote patient monitoring, virtual consultations, and digital data collection that expand trial accessibility and reduce geographic barriers. Digital biomarkers derived from wearable devices, smartphone sensors, and other connected health technologies are providing new endpoints and outcome measures for clinical trials.
Real-world evidence integration is becoming increasingly important as AI systems analyze large-scale patient data from electronic health records, insurance claims, and patient registries to supplement traditional clinical trial data. Precision medicine approaches are driving demand for AI technologies capable of identifying patient subgroups most likely to respond to specific treatments.
Regulatory technology adoption is accelerating as health authorities implement AI tools for more efficient drug review processes and regulatory decision-making. Cloud-based platforms are becoming the preferred deployment model for AI clinical trial solutions, offering scalability, cost-effectiveness, and easier integration with existing systems.
Collaborative partnerships between pharmaceutical companies, technology firms, and academic institutions are creating new business models and accelerating AI development in clinical research. MWR data indicates that strategic partnerships in this sector have increased by 40% over the past two years.
Recent industry developments highlight the rapid evolution of AI technologies in clinical trials and increasing industry adoption. Major pharmaceutical companies are establishing dedicated AI centers of excellence and investing billions in digital transformation initiatives focused on clinical research optimization.
Regulatory milestones include the FDA’s publication of guidance documents for AI/ML-based medical devices and the EMA’s establishment of innovation task forces to support AI technology assessment. Technology breakthroughs in natural language processing, computer vision, and predictive analytics are enabling new applications in clinical trial management and optimization.
Strategic acquisitions and partnerships are reshaping the competitive landscape as established healthcare companies acquire AI startups and technology firms expand their healthcare portfolios. Investment activity continues to be robust, with venture capital and private equity firms providing substantial funding for AI clinical trial technology development.
Academic collaborations are advancing AI research in clinical trials through partnerships between universities, pharmaceutical companies, and technology firms. International initiatives are promoting global standards for AI implementation in clinical research and facilitating cross-border collaboration.
Strategic recommendations for market participants include focusing on comprehensive platform development rather than point solutions, as integrated AI systems provide greater value and competitive differentiation. Partnership strategies should emphasize collaboration with established pharmaceutical companies and clinical research organizations to accelerate market penetration and technology validation.
Investment priorities should focus on data quality and integration capabilities, as the effectiveness of AI systems depends heavily on access to high-quality, diverse datasets. Regulatory engagement is crucial for companies developing AI clinical trial technologies, requiring proactive collaboration with health authorities to ensure compliance and facilitate approval processes.
Talent acquisition and retention strategies should prioritize building teams with combined expertise in AI technology, clinical research, and regulatory affairs. Geographic expansion opportunities should be evaluated based on regulatory environment, market maturity, and local partnership potential.
Technology development should emphasize explainable AI capabilities that provide transparency in decision-making processes, addressing regulatory and user acceptance concerns. Customer success programs are essential for ensuring successful AI implementation and demonstrating measurable value to pharmaceutical and biotechnology clients.
Future market prospects for AI in clinical trials remain highly positive, with continued growth expected across all major segments and geographic regions. Technology advancement will likely focus on developing more sophisticated AI models capable of handling complex clinical research challenges and providing deeper insights into patient populations and treatment responses.
Regulatory evolution is expected to continue supporting AI adoption through clearer guidance documents, streamlined approval processes, and international harmonization efforts. Market consolidation may accelerate as successful AI companies are acquired by larger healthcare organizations seeking to expand their digital capabilities.
Integration trends will likely see AI clinical trial technologies becoming standard components of comprehensive clinical research platforms rather than standalone solutions. Global expansion opportunities will continue to emerge as healthcare infrastructure develops in emerging markets and regulatory frameworks mature.
Innovation focus will shift toward developing AI systems capable of supporting increasingly complex clinical trial designs, including adaptive trials, master protocols, and precision medicine approaches. MarkWide Research projects that AI adoption rates in clinical trials will continue accelerating, with implementation expected to become standard practice across the pharmaceutical industry within the next decade.
The AI in clinical trials market represents a transformative force in pharmaceutical research and development, offering unprecedented opportunities to improve trial efficiency, reduce costs, and accelerate the delivery of new treatments to patients. Market fundamentals remain strong, supported by increasing industry adoption, regulatory acceptance, and demonstrated value in real-world implementations.
Growth prospects continue to be favorable across all major market segments, with particular strength in patient recruitment, protocol optimization, and data management applications. Technological advancement and increasing data availability are creating new opportunities for AI innovation in clinical research, while regulatory support is facilitating broader market adoption.
Strategic positioning in this market requires a comprehensive understanding of clinical research workflows, regulatory requirements, and technology capabilities. Success factors include developing integrated solutions, establishing strong industry partnerships, and maintaining focus on measurable value delivery to pharmaceutical and biotechnology clients. The AI in clinical trials market is poised for continued expansion as the healthcare industry embraces digital transformation and seeks innovative approaches to address the challenges of modern drug development.
What is AI In Clinical Trials?
AI in clinical trials refers to the application of artificial intelligence technologies to enhance the efficiency and effectiveness of clinical research. This includes data analysis, patient recruitment, and trial monitoring, ultimately aiming to improve outcomes and reduce costs.
What are the key companies in the AI In Clinical Trials Market?
Key companies in the AI in clinical trials market include IBM Watson Health, Medidata Solutions, and Oracle Health Sciences, among others. These companies are leveraging AI to streamline processes and improve data management in clinical research.
What are the drivers of growth in the AI In Clinical Trials Market?
The growth of the AI in clinical trials market is driven by the increasing complexity of clinical trials, the need for faster drug development, and the rising volume of data generated in research. Additionally, advancements in machine learning and data analytics are facilitating more efficient trial designs.
What challenges does the AI In Clinical Trials Market face?
Challenges in the AI in clinical trials market include data privacy concerns, regulatory hurdles, and the integration of AI technologies with existing systems. These factors can hinder the adoption of AI solutions in clinical research.
What opportunities exist in the AI In Clinical Trials Market?
Opportunities in the AI in clinical trials market include the potential for personalized medicine, improved patient engagement through AI-driven tools, and the ability to analyze large datasets for better decision-making. These advancements can lead to more effective and targeted therapies.
What trends are shaping the AI In Clinical Trials Market?
Trends in the AI in clinical trials market include the increasing use of real-world data, the rise of decentralized clinical trials, and the integration of AI with wearable technology. These trends are transforming how clinical trials are conducted and enhancing patient participation.
AI In Clinical Trials Market
| Segmentation Details | Description |
|---|---|
| Product Type | Data Management, Patient Recruitment, Trial Monitoring, Analytics Tools |
| End User | Pharmaceutical Companies, Biotechnology Firms, Research Institutions, Contract Research Organizations |
| Technology | Machine Learning, Natural Language Processing, Predictive Analytics, Cloud Computing |
| Application | Patient Engagement, Risk Assessment, Data Analysis, Compliance Management |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the AI In Clinical Trials Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at