444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The circulating biomarkers market has been experiencing significant growth in recent years. Biomarkers, as measurable indicators of biological processes or conditions, are found in various bodily fluids such as blood, urine, and saliva. These biomarkers provide valuable insights into the presence, progression, and response to diseases, making them essential tools for diagnostics, prognostics, and monitoring. The global market for circulating biomarkers encompasses a wide range of industries, including healthcare, pharmaceuticals, biotechnology, and research.
Meaning
Circulating biomarkers refer to biomolecules or substances that are present in the bloodstream or other bodily fluids. These biomarkers can include proteins, nucleic acids, cells, exosomes, and metabolites. They are released into circulation by normal physiological processes or as a result of disease-related changes. The analysis of circulating biomarkers offers valuable information on disease diagnosis, prognosis, and treatment response, enabling personalized medicine approaches.
Executive Summary
The executive summary provides a concise overview of the circulating biomarkers market. It highlights the key market trends, growth drivers, and challenges faced by industry participants. The summary also provides an insight into the regional analysis, competitive landscape, and segmentation of the market. Additionally, it discusses the impact of COVID-19 on the market and presents future outlook and analyst suggestions.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
The global rise in cancer prevalence and the growing adoption of non-invasive diagnostic methods are major drivers fueling the circulating biomarkers market.
Liquid biopsy technologies have revolutionized the detection of ctDNA, exosomes, and miRNAs, enabling early diagnosis and real-time monitoring of treatment response.
Increasing regulatory approvals for biomarker-based companion diagnostics are enhancing market credibility and clinical adoption.
Integration of next-generation sequencing (NGS) and digital PCR technologies has significantly improved biomarker detection sensitivity and specificity.
Pharmaceutical companies are leveraging circulating biomarkers for stratifying patients in clinical trials, enhancing trial efficiency and drug development success rates.
Market Drivers
Several factors are contributing to the expansion of the Circulating Biomarkers market:
Growing Burden of Chronic Diseases: Rising global incidences of cancer, cardiovascular disorders, and neurodegenerative conditions are driving demand for minimally invasive diagnostic tools.
Advancements in Liquid Biopsy: Breakthroughs in blood-based diagnostics have made it possible to detect disease-related biomarkers without tissue biopsies.
Precision Medicine Initiatives: The shift towards personalized medicine is increasing the need for real-time, patient-specific biomarker profiles to tailor therapies.
Research and Development Investments: Both public and private sector funding in biomarker discovery and validation are accelerating market growth.
Companion Diagnostics Growth: Circulating biomarkers are increasingly being used as companion diagnostics for targeted therapies, enabling better patient outcomes.
Market Restraints
Despite its growth potential, the Circulating Biomarkers market faces several constraints:
Analytical Challenges: Standardization and validation of biomarker assays remain challenging, impacting reproducibility and clinical utility.
High Development Costs: The development of biomarker-based diagnostics involves extensive research, clinical trials, and regulatory approvals, which can be cost-prohibitive.
Limited Awareness: In some regions, limited awareness among healthcare providers and patients about circulating biomarkers restricts adoption.
Regulatory Hurdles: Varying regulatory requirements across countries can slow down the approval and commercialization of biomarker-based products.
Ethical and Data Security Issues: Handling of genomic data raises privacy concerns, especially in personalized medicine applications.
Market Opportunities
The Circulating Biomarkers market presents substantial opportunities for expansion and innovation:
Expansion Beyond Oncology: While oncology dominates the market, increasing applications in cardiovascular, infectious, and autoimmune diseases present untapped growth avenues.
AI and Big Data Integration: Advanced analytics can enhance biomarker interpretation, aiding in pattern recognition, disease prediction, and therapy optimization.
Point-of-Care Testing Development: Miniaturized, rapid diagnostic devices for biomarker detection can increase accessibility in remote and resource-limited settings.
Biomarker-based Drug Development: Pharmaceutical companies are increasingly incorporating biomarkers in early drug development to improve clinical trial outcomes.
Strategic Collaborations: Partnerships between diagnostic firms, biotech companies, and academic institutions can accelerate innovation and market penetration.
Market Dynamics
The circulating biomarkers market is characterized by dynamic factors that influence its growth and development. These dynamics include market drivers, restraints, opportunities, and challenges that shape the industry landscape. The market dynamics are influenced by technological advancements, regulatory frameworks, competitive landscape, and changing healthcare needs. Continuous innovation, strategic collaborations, and market expansion efforts are key strategies adopted by industry players to thrive in this dynamic market.
Regional Analysis
Competitive Landscape
Leading Companies in the Circulating Biomarkers Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
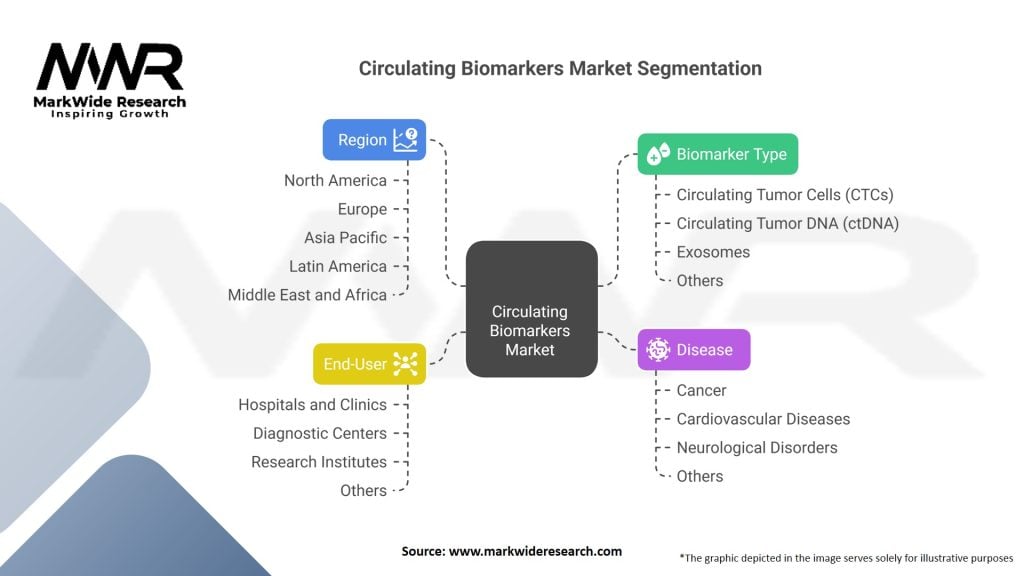
Segmentation
The circulating biomarkers market can be segmented based on various parameters, including biomarker type, application, end-user, and region.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
The circulating biomarkers market can be analyzed using a SWOT (Strengths, Weaknesses, Opportunities, and Threats) framework:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the circulating biomarkers market. Here are some key observations:
Key Industry Developments
Analyst Suggestions
Future Outlook
The circulating biomarkers market is expected to witness significant growth in the coming years. The increasing prevalence of chronic diseases, advancements in technology, and the shift towards personalized medicine are driving market expansion. The integration of AI, the emergence of liquid biopsy applications, and the focus on companion diagnostics present substantial opportunities for market players. However, challenges related to standardization, validation, and regulatory frameworks need to be addressed. Overall, the future outlook for the circulating biomarkers market is promising, with continued advancements in research, technology, and industry collaborations.
Conclusion
The circulating biomarkers market is a dynamic and rapidly evolving field with immense potential for disease diagnosis, prognosis, and treatment monitoring. Biomarkers provide valuable insights into the presence, progression, and response to diseases, enabling personalized medicine approaches. The market is driven by the increasing prevalence of chronic diseases, advancements in technology, and the shift towards personalized medicine. However, challenges such as standardization, validation, and regulatory frameworks need to be addressed to realize the full potential of circulating biomarkers. The future outlook for the market is optimistic, with opportunities arising from emerging applications, technological advancements, and strategic collaborations. The market is poised for continued growth, contributing to improved patient care and outcomes in various disease areas.
Circulating Biomarkers Market
| Segmentation | Details |
|---|---|
| Biomarker Type | Circulating Tumor Cells (CTCs), Circulating Tumor DNA (ctDNA), Exosomes, Others |
| Disease | Cancer, Cardiovascular Diseases, Neurological Disorders, Others |
| End-User | Hospitals and Clinics, Diagnostic Centers, Research Institutes, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Circulating Biomarkers Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at