444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The cardiac biomarkers testing market has witnessed significant growth in recent years, driven by the increasing prevalence of cardiovascular diseases and the growing demand for early diagnosis and treatment. Cardiac biomarkers are substances released into the bloodstream in response to heart damage or stress, and their measurement provides valuable information for the diagnosis, prognosis, and monitoring of various cardiac conditions. The market for cardiac biomarkers testing encompasses a wide range of diagnostic tests and technologies used to detect and quantify these biomarkers.
Meaning
Cardiac biomarkers testing refers to the process of measuring specific substances or molecules in the blood that are released when the heart is damaged or under stress. These biomarkers can indicate the presence and severity of various heart conditions, such as myocardial infarction (heart attack), heart failure, and coronary artery disease. The measurement of cardiac biomarkers plays a crucial role in the early detection, diagnosis, and management of cardiovascular diseases.
Executive Summary
The cardiac biomarkers testing market is witnessing substantial growth due to the increasing incidence of cardiovascular diseases worldwide. The market offers a wide range of diagnostic tests and technologies for the measurement of cardiac biomarkers, enabling healthcare providers to make accurate and timely decisions regarding patient care. Key players in the market are focusing on product development and strategic collaborations to enhance their market presence and gain a competitive edge.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The cardiac biomarkers testing market is dynamic, influenced by various factors such as technological advancements, disease prevalence, regulatory landscape, and patient demographics. The market is characterized by intense competition among key players, with a focus on product innovation, strategic partnerships, and geographical expansion. Additionally, collaborations between diagnostic companies and research institutions contribute to advancements in cardiac biomarkers testing. The market dynamics are further influenced by changing healthcare policies, reimbursement systems, and evolving patient preferences.
Regional Analysis
The cardiac biomarkers testing market exhibits regional variations due to differences in disease prevalence, healthcare infrastructure, regulatory frameworks, and economic factors. North America dominates the market, driven by the high prevalence of cardiovascular diseases, well-established healthcare systems, and technological advancements. Europe follows closely, with a significant emphasis on preventive healthcare and early disease detection. The Asia-Pacific region presents lucrative growth opportunities due to the rising healthcare expenditure, increasing geriatric population, and growing awareness about cardiovascular diseases. Latin America and the Middle East & Africa region are also witnessing steady market growth, driven by improving healthcare infrastructure and increasing access to diagnostic services.
Competitive Landscape
Leading Companies in the Cardiac Biomarkers Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

Segmentation
The cardiac biomarkers testing market can be segmented based on the type of biomarker, test type, end-user, and geography. Based on the type of biomarker, the market can be segmented into troponin, CK-MB, BNP/NT-proBNP, myoglobin, and others. Troponin biomarkers dominate the market due to their high sensitivity and specificity in detecting cardiac damage. Based on the test type, the market can be categorized into laboratory-based tests and point-of-care tests. Laboratory-based tests are commonly performed in centralized clinical laboratories, while point-of-care tests offer rapid results at or near the patient’s bedside.
End-users of cardiac biomarkers testing include hospitals, diagnostic laboratories, academic and research institutions, and ambulatory care centers. Hospitals account for the largest market share, given the high patient footfall and the availability of specialized cardiac care facilities. Geographically, the market can be segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America currently holds the largest market share, while the Asia-Pacific region is expected to witness significant growth during the forecast period.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had a significant impact on the cardiac biomarkers testing market. While the immediate focus has been on diagnosing and managing COVID-19 cases, the pandemic has also disrupted routine healthcare services, including cardiac care. The reduced access to healthcare facilities, delayed elective procedures, and altered patient behavior have affected the demand for cardiac biomarkers testing.
However, the pandemic has also highlighted the importance of cardiac biomarkers in assessing the impact of COVID-19 on the cardiovascular system. Biomarkers such as troponin and BNP have been used to identify myocardial injury and cardiac dysfunction in COVID-19 patients. This increased focus on cardiac biomarkers testing in the context of COVID-19 may drive further research and advancements in the field.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the cardiac biomarkers testing market looks promising, driven by the increasing prevalence of cardiovascular diseases, advancements in diagnostic technologies, and a growing focus on personalized medicine. The market is expected to witness continued product innovation, with a focus on high-sensitivity and rapid diagnostic tests. Additionally, the integration of artificial intelligence, big data analytics, and multi-omics approaches holds potential for improving diagnostic accuracy and patient outcomes. Expansion in emerging markets and collaborations between industry players and research institutions will play a crucial role in shaping the future of the market.
Conclusion
The cardiac biomarkers testing market is experiencing significant growth, driven by the rising prevalence of cardiovascular diseases and the demand for early diagnosis and personalized treatment. Technological advancements, such as high-sensitivity troponin assays and point-of-care testing devices, are revolutionizing the field, enabling accurate and rapid diagnostic results. While the market faces challenges such as high costs and lack of standardization, opportunities exist in the form of growing demand for point-of-care testing, expansion in emerging markets, and the integration of artificial intelligence and big data analytics. The future of the market looks promising, with continued product innovation, strategic collaborations, and a focus on personalized medicine and digital health solutions.
What is Cardiac Biomarkers Testing?
Cardiac biomarkers testing refers to the measurement of specific proteins or substances in the blood that indicate heart muscle damage or stress. These tests are crucial for diagnosing conditions such as myocardial infarction and heart failure.
What are the key players in the Cardiac Biomarkers Testing market?
Key players in the Cardiac Biomarkers Testing market include Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers, among others. These companies are known for their innovative diagnostic solutions and extensive product portfolios in cardiac care.
What are the main drivers of the Cardiac Biomarkers Testing market?
The main drivers of the Cardiac Biomarkers Testing market include the increasing prevalence of cardiovascular diseases, advancements in biomarker research, and the growing demand for rapid and accurate diagnostic tools. These factors contribute to the expansion of testing services in healthcare settings.
What challenges does the Cardiac Biomarkers Testing market face?
The Cardiac Biomarkers Testing market faces challenges such as the high cost of advanced testing technologies and the need for regulatory approvals. Additionally, variability in biomarker levels among different populations can complicate test interpretations.
What opportunities exist in the Cardiac Biomarkers Testing market?
Opportunities in the Cardiac Biomarkers Testing market include the development of novel biomarkers and point-of-care testing solutions. There is also potential for growth in emerging markets where healthcare infrastructure is improving.
What trends are shaping the Cardiac Biomarkers Testing market?
Trends shaping the Cardiac Biomarkers Testing market include the integration of artificial intelligence in diagnostic processes and the increasing focus on personalized medicine. These trends aim to enhance the accuracy and efficiency of cardiac assessments.
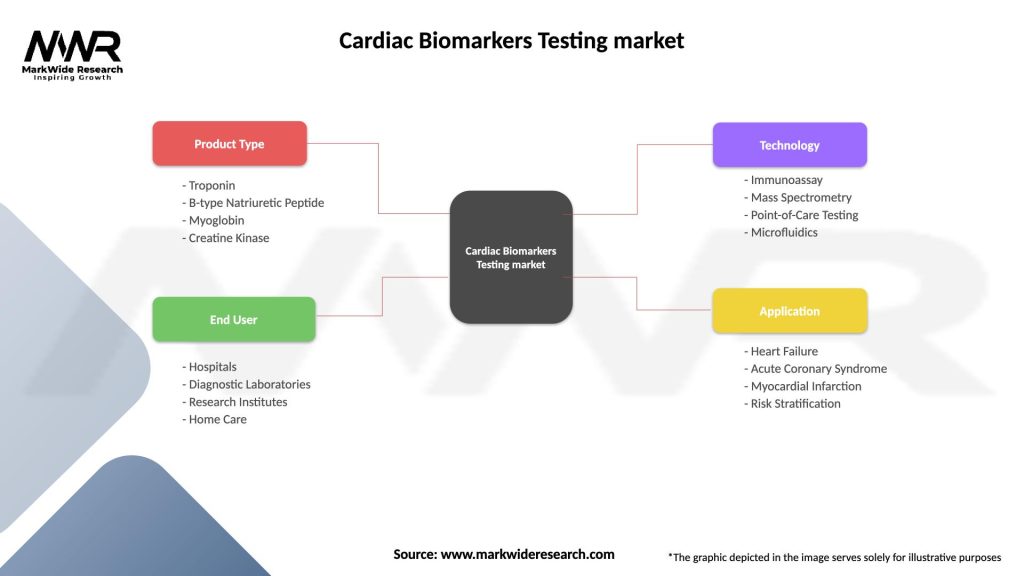
Cardiac Biomarkers Testing market
| Segmentation Details | Description |
|---|---|
| Product Type | Troponin, B-type Natriuretic Peptide, Myoglobin, Creatine Kinase |
| End User | Hospitals, Diagnostic Laboratories, Research Institutes, Home Care |
| Technology | Immunoassay, Mass Spectrometry, Point-of-Care Testing, Microfluidics |
| Application | Heart Failure, Acute Coronary Syndrome, Myocardial Infarction, Risk Stratification |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Cardiac Biomarkers Testing Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at