444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The RNA-based therapeutic market has witnessed significant growth in recent years, driven by advancements in biotechnology and the increasing demand for targeted and personalized therapies. RNA-based therapeutics, which include RNA interference (RNAi) and antisense oligonucleotides (ASOs), offer promising opportunities for the treatment of various diseases, including cancer, genetic disorders, and infectious diseases. These therapeutics work by modulating gene expression and protein synthesis, providing a novel approach to address unmet medical needs. As a result, the market for RNA-based therapeutics has gained traction and is projected to expand further in the coming years.
Meaning
RNA-based therapeutics refer to a class of drugs that utilize RNA molecules to target specific genes and regulate their expression. These therapeutics can be broadly categorized into RNA interference (RNAi) and antisense oligonucleotides (ASOs). RNAi-based therapeutics use small interfering RNA (siRNA) molecules to silence disease-causing genes, while ASOs are short synthetic DNA or RNA molecules that can selectively bind to RNA molecules and modulate their function. By targeting specific genes or RNA sequences, RNA-based therapeutics can disrupt disease pathways and potentially offer effective treatments for a wide range of diseases.
Executive Summary
The RNA-based therapeutic market has witnessed significant growth in recent years, driven by advancements in biotechnology and the increasing demand for targeted and personalized therapies. The market is expected to continue its upward trajectory, fueled by factors such as the rising prevalence of chronic diseases, increasing investment in research and development activities, and growing adoption of precision medicine approaches. However, the market also faces challenges such as the high cost of development and regulatory complexities. Despite these challenges, the potential benefits of RNA-based therapeutics make them a promising avenue for future medical advancements.
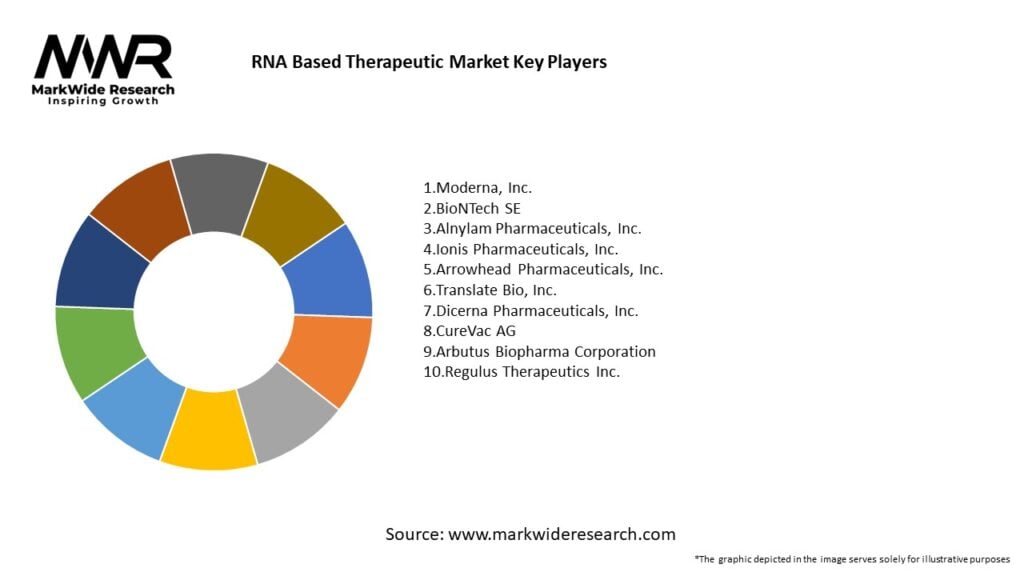
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The RNA-based therapeutic market is driven by a combination of factors, including the rising prevalence of chronic diseases, advancements in biotechnology, increasing investment in research and development, and the growing demand for personalized medicine. These drivers are supported by key market dynamics, such as regulatory considerations, the competitive landscape, and regional variations in market growth.
Regional Analysis
The global RNA-based therapeutic market is geographically segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America currently dominates the market, primarily driven by the presence of well-established biotechnology companies, robust research infrastructure, and favorable regulatory frameworks. The region also benefits from high healthcare expenditure and increasing adoption of precision medicine approaches. Europe is the second-largest market for RNA-based therapeutics, with significant contributions from countries such as Germany, the United Kingdom, and France. The Asia Pacific region is expected to witness rapid market growth in the forecast period, fueled by increasing healthcare expenditure, a growing pharmaceutical industry, and rising awareness about personalized medicine. Latin America and the Middle East and Africa offer untapped potential, driven by the increasing burden of chronic diseases and efforts to improve healthcare access in these regions.
Competitive Landscape
Leading Companies in the RNA Based Therapeutic Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.

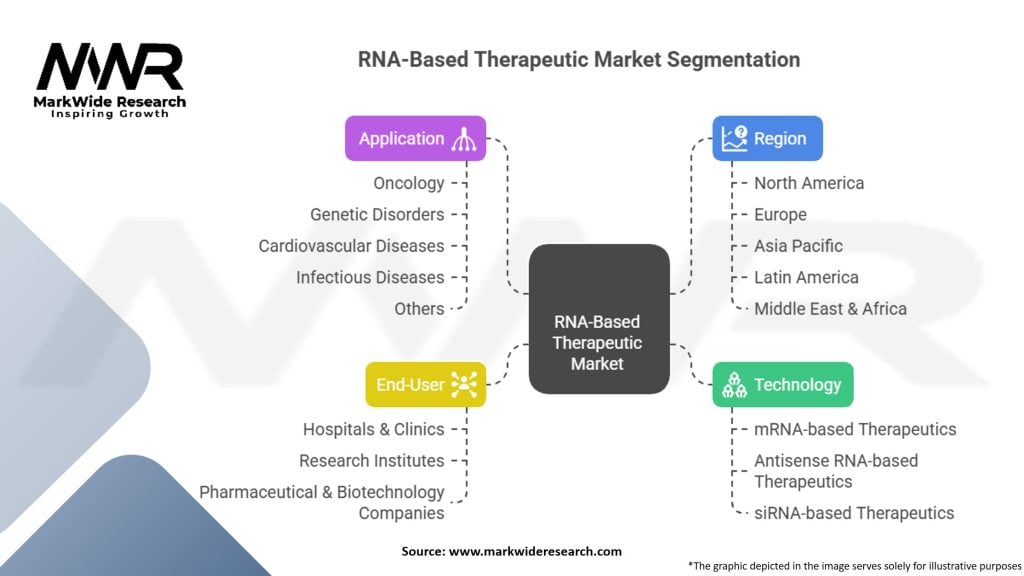
Segmentation
The RNA-based therapeutic market can be segmented based on technology, application, and end-user.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has had both positive and negative impacts on the RNA-based therapeutic market. On the positive side, the pandemic has accelerated research and development efforts in the field of RNA-based vaccines. The successful development and emergency use authorization of mRNA-based COVID-19 vaccines have showcased the potential of RNA-based therapeutics in generating rapid immune responses and addressing infectious diseases.
However, the pandemic has also posed challenges for ongoing clinical trials and disrupted supply chains, affecting the progress of RNA-based therapeutic development in other disease areas. The diversion of resources and focus towards COVID-19-related research may have temporarily impacted the overall advancement of RNA-based therapeutics. Nonetheless, the lessons learned from the pandemic and the advancements made in mRNA vaccine technology are expected to have long-term positive effects on the market, driving further investments and advancements in RNA-based therapeutics.
Key Industry Developments
Analyst Suggestions
Future Outlook
The future of the RNA-based therapeutic market appears promising, driven by advancements in biotechnology, increasing prevalence of chronic diseases, and the demand for personalized medicine. Continued investment in research and development, along with strategic collaborations, will contribute to the development of innovative RNA-based therapeutics.
Further advancements in delivery systems, including the utilization of nanotechnology and gene editing technologies, will enhance the specificity and efficacy of RNA-based therapeutics. Expansion into rare diseases and emerging markets will unlock new growth opportunities. Additionally, the lessons learned from the COVID-19 pandemic and the success of mRNA-based vaccines will likely accelerate the development of RNA-based therapeutics for various diseases.
However, challenges such as high development costs, regulatory complexities, and the need for clinical validation remain. Overcoming these challenges will require sustained efforts from industry stakeholders and collaboration with regulatory authorities to create a favorable environment for the development and commercialization of RNA-based therapeutics.
Conclusion
The RNA-based therapeutic market is witnessing significant growth, driven by advancements in biotechnology, the rising prevalence of chronic diseases, and the demand for targeted and personalized therapies. RNA-based therapeutics offer the potential to modulate gene expression and provide effective treatments for a wide range of diseases, including cancer, genetic disorders, and infectious diseases.
While the market presents immense opportunities, challenges such as high development costs, regulatory complexities, and the need for clinical validation exist. Overcoming these challenges requires continued investment in research and development, strategic collaborations, and advancements in delivery systems.
Looking ahead, the future of the RNA-based therapeutic market is promising. Ongoing innovations, increased understanding of disease mechanisms, and the convergence of technologies like gene editing will further enhance the therapeutic potential of RNA-based therapeutics. The market is poised for continued growth, driven by the pursuit of precision medicine and the development of transformative treatments for patients worldwide.
What is RNA Based Therapeutic?
RNA Based Therapeutics are a class of treatments that utilize RNA molecules to modulate gene expression and protein synthesis. These therapies can target various diseases, including genetic disorders and cancers, by delivering RNA to cells to influence their behavior.
What are the key companies in the RNA Based Therapeutic Market?
Key companies in the RNA Based Therapeutic Market include Moderna, BioNTech, and Alnylam Pharmaceuticals, which are known for their innovative RNA-based therapies and vaccines. These companies are at the forefront of research and development in this rapidly evolving field, among others.
What are the growth factors driving the RNA Based Therapeutic Market?
The RNA Based Therapeutic Market is driven by advancements in RNA technology, increasing prevalence of genetic disorders, and the growing demand for personalized medicine. Additionally, the success of mRNA vaccines during health crises has accelerated interest and investment in RNA therapeutics.
What challenges does the RNA Based Therapeutic Market face?
Challenges in the RNA Based Therapeutic Market include issues related to delivery mechanisms, stability of RNA molecules, and regulatory hurdles. Ensuring effective delivery to target cells while minimizing side effects remains a significant obstacle for developers.
What future opportunities exist in the RNA Based Therapeutic Market?
Future opportunities in the RNA Based Therapeutic Market include the development of novel therapies for rare diseases and the potential for combination therapies that integrate RNA-based treatments with other modalities. The ongoing research into RNA editing technologies also presents exciting possibilities.
What trends are shaping the RNA Based Therapeutic Market?
Trends in the RNA Based Therapeutic Market include the increasing focus on RNA interference (RNAi) technologies and the expansion of mRNA vaccine applications beyond infectious diseases. Additionally, collaborations between biotech firms and academic institutions are fostering innovation in this space.
RNA Based Therapeutic Market
| Segmentation | Details |
|---|---|
| Technology | mRNA-based Therapeutics, Antisense RNA-based Therapeutics, siRNA-based Therapeutics |
| Application | Oncology, Genetic Disorders, Cardiovascular Diseases, Infectious Diseases, Others |
| End-User | Hospitals & Clinics, Research Institutes, Pharmaceutical & Biotechnology Companies |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the RNA Based Therapeutic Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at