444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Global Biosimilar Testing and Development Services Market are at the forefront of biopharmaceutical innovation, enabling the development and commercialization of biosimilar drugs that offer cost-effective alternatives to branded biologics. These services encompass a wide range of analytical and developmental activities critical for demonstrating biosimilarity, ensuring patient safety, and gaining regulatory approval. In this comprehensive guide, we explore the meaning, executive summary, key market insights, and future outlook of the Biosimilar Testing and Development Services market, providing essential information for industry participants and stakeholders.
Meaning
Biosimilar Testing and Development Services refer to the comprehensive suite of services offered by specialized contract research organizations (CROs) and laboratories to support the development of biosimilar drugs. These services encompass analytical testing, comparability studies, non-clinical and clinical assessments, and regulatory submission support. Biosimilar testing and development are crucial for demonstrating the quality, safety, and efficacy of biosimilar products, paving the way for their approval and commercialization.
Executive Summary
The Global Biosimilar Testing and Development Services Market are experiencing rapid growth, driven by the increasing demand for biosimilars, the complexity of biologics, and the need for specialized expertise. This executive summary provides a snapshot of key trends, market drivers, restraints, and opportunities in this sector. It offers a concise overview of market dynamics, the competitive landscape, segmentation, and the impact of external factors. Additionally, it outlines future prospects and provides analyst suggestions to guide industry participants and stakeholders.
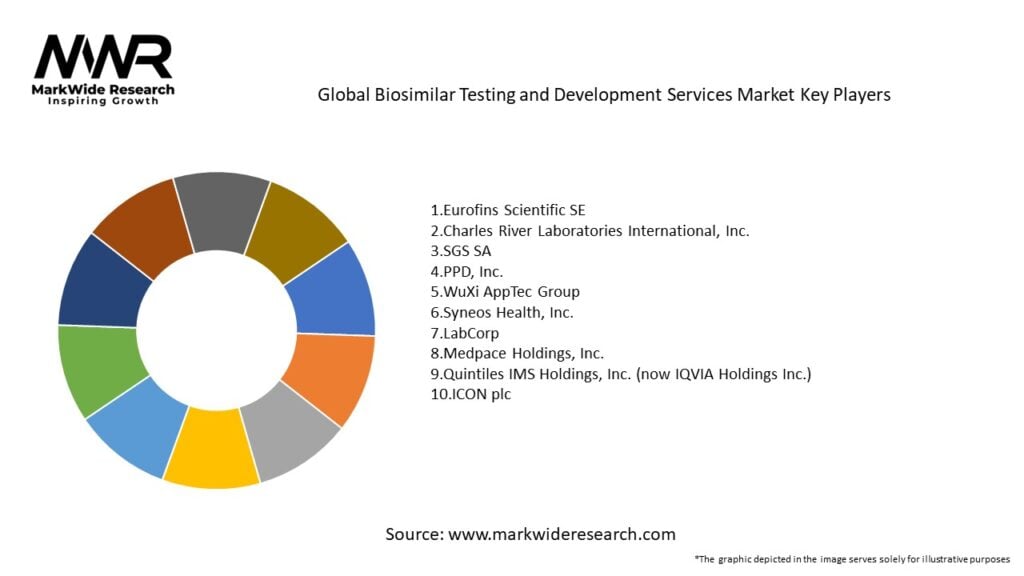
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
The Global Biosimilar Testing and Development Services Market is influenced by several key factors:
Market Drivers
Several factors are driving the growth of the Global Biosimilar Testing and Development Services Market:
Market Restraints
Despite the promising growth prospects, the Global Biosimilar Testing and Development Services Market faces several challenges:
Market Opportunities
The Global Biosimilar Testing and Development Services Market presents numerous opportunities for growth:
Market Dynamics
The dynamics of the Global Biosimilar Testing and Development Services Market are shaped by both supply-side and demand-side factors:
Regional Analysis
The Global Biosimilar Testing and Development Services Market is experiencing different growth trends across various regions:
Competitive Landscape
Leading companies in the Global Biosimilar Testing and Development Services Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
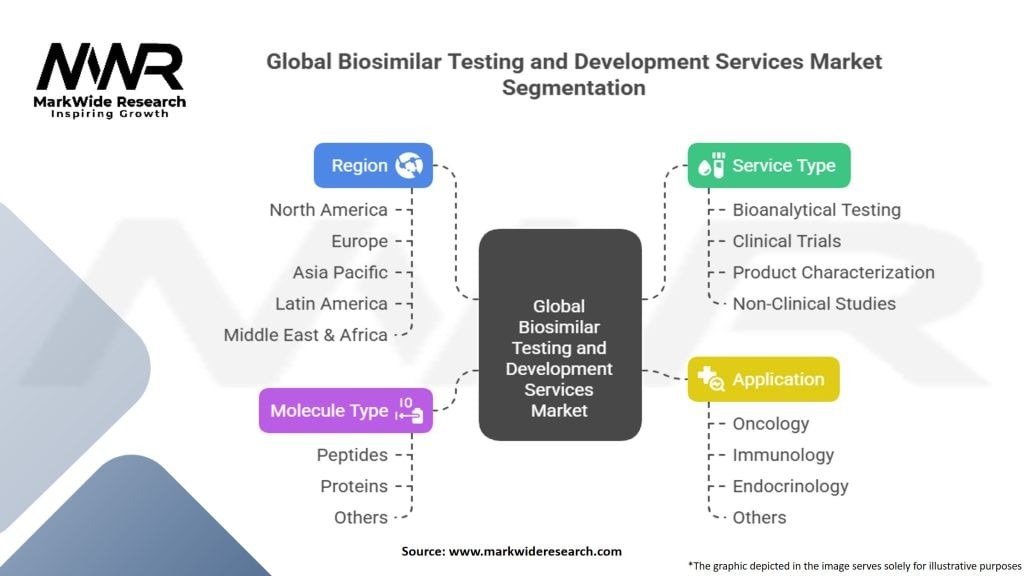
Segmentation
The Global Biosimilar Testing and Development Services Market can be segmented based on various factors:
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Weaknesses:
Opportunities:
Threats:
Market Key Trends
COVID-19 Impact
The COVID-19 pandemic has had far-reaching effects on industries worldwide, including the Biosimilar Testing and Development Services market. This section examines how the pandemic has influenced market dynamics, clinical trial timelines, and supply chain disruptions. It also discusses the resilience and adaptability demonstrated by service providers during this challenging period.
Key Industry Developments
The Biosimilar Testing and Development Services market is characterized by continuous innovation and technological advancements. This section highlights key industry developments, including the expansion of biosimilar pipelines, collaborations with biosimilar developers, and research breakthroughs that have shaped the market’s trajectory. These developments provide valuable insights into the direction the industry is heading.
Analyst Suggestions
In a dynamic and competitive market like Biosimilar Testing and Development Services, expert guidance is invaluable. This section offers suggestions and recommendations for service providers, biosimilar developers, and other stakeholders. Whether it’s optimizing analytical methods, enhancing regulatory expertise, or fostering partnerships to streamline development, these insights can help pave the way for success.
Future Outlook
The Biosimilar Testing and Development Services market is poised for continued growth and innovation. In this section, we look ahead to the future of the market, considering emerging trends, technological advancements, and evolving regulatory pathways. Service providers, biosimilar developers, and stakeholders can use this information to formulate long-term strategies and contribute to the ongoing advancement of biosimilar innovation, ultimately benefiting patients and healthcare systems worldwide.
Conclusion
In conclusion, the Global Biosimilar Testing and Development Services Market plays a vital role in advancing biopharmaceutical innovation and expanding access to cost-effective biologic therapies. Despite the challenges it faces, the market offers significant opportunities for growth and contribution to improved healthcare worldwide. With the right strategies and a keen understanding of market dynamics, service providers, biosimilar developers, and stakeholders can continue to drive the development and commercialization of biosimilar drugs, ultimately benefiting patients and fostering a more accessible and sustainable biopharmaceutical landscape.
What is Biosimilar Testing and Development Services?
Biosimilar Testing and Development Services refer to the processes and methodologies used to evaluate and develop biosimilar products, which are biologic medical products highly similar to already approved reference products. These services encompass various stages, including preclinical testing, clinical trials, and regulatory compliance.
What are the key players in the Global Biosimilar Testing and Development Services Market?
Key players in the Global Biosimilar Testing and Development Services Market include companies like Samsung BioLogics, Lonza Group, and Catalent, which provide comprehensive testing and development services for biosimilars, among others.
What are the growth factors driving the Global Biosimilar Testing and Development Services Market?
The growth of the Global Biosimilar Testing and Development Services Market is driven by factors such as the increasing prevalence of chronic diseases, the rising demand for cost-effective biologics, and advancements in biopharmaceutical technologies.
What challenges does the Global Biosimilar Testing and Development Services Market face?
Challenges in the Global Biosimilar Testing and Development Services Market include stringent regulatory requirements, the complexity of biosimilar development, and the need for extensive clinical data to demonstrate similarity to reference products.
What opportunities exist in the Global Biosimilar Testing and Development Services Market?
Opportunities in the Global Biosimilar Testing and Development Services Market include the potential for increased collaboration between biopharmaceutical companies and testing service providers, as well as the expansion of biosimilar applications in various therapeutic areas such as oncology and autoimmune diseases.
What trends are shaping the Global Biosimilar Testing and Development Services Market?
Trends in the Global Biosimilar Testing and Development Services Market include the growing focus on personalized medicine, the integration of advanced technologies like artificial intelligence in drug development, and an increasing emphasis on sustainability in biomanufacturing processes.
Global Biosimilar Testing and Development Services Market
| Segmentation Details | Information |
|---|---|
| Service Type | Bioanalytical Testing, Clinical Trials, Product Characterization, Non-Clinical Studies |
| Molecule Type | Peptides, Proteins, Others |
| Application | Oncology, Immunology, Endocrinology, Others |
| Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Global Biosimilar Testing and Development Services Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at