444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Alexipharmic Drugs Market refers to the pharmaceutical segment focused on the development, production, and distribution of drugs that have antidotal properties. These drugs are designed to counteract the effects of various toxins, poisons, or harmful substances in the body. The market for alexipharmic drugs plays a crucial role in emergency medical care, poison control centers, and other healthcare settings where toxic exposures and overdoses occur. The market encompasses a wide range of antidote medications and therapeutic interventions aimed at saving lives and minimizing the detrimental effects of toxins on the human body.
Meaning
Alexipharmic drugs, also known as antidotes or antidotal drugs, are substances or medications used to counteract the toxic effects of poisons or harmful substances. They work by neutralizing or blocking the toxic effects of the offending substance, enhancing elimination from the body, or providing supportive care to the affected individual. These drugs are typically administered in emergency situations to prevent or minimize the severity of poisoning or overdose.
Executive Summary
The global market for Alexipharmic drugs is driven by the increasing incidence of poisonings and toxicological emergencies, the growing awareness about the importance of prompt and effective treatment, and advancements in drug development and technology. The market presents significant opportunities for pharmaceutical companies, healthcare providers, and other stakeholders to develop innovative antidotes, improve access to treatment, and enhance patient outcomes.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Alexipharmic drugs market is characterized by dynamic factors that influence its growth and development. These dynamics include market drivers, restraints, opportunities, and trends that shape the industry landscape. Understanding the market dynamics is crucial for stakeholders to make informed decisions and strategize effectively.
Regional Analysis
The Alexipharmic drugs market exhibits regional variations influenced by factors such as the prevalence of poisonings, healthcare infrastructure, regulatory frameworks, and economic factors. A comprehensive regional analysis helps identify opportunities, challenges, and growth potential in specific geographic areas.
Competitive Landscape
Leading companies in the Alexipharmic Drugs market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
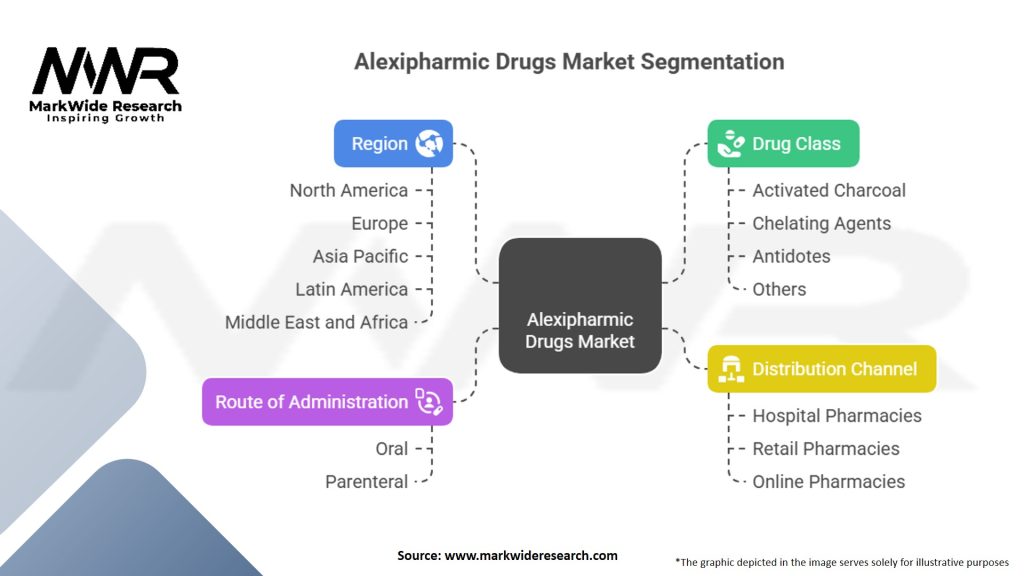
Segmentation
The market can be segmented based on various factors such as drug type, route of administration, end-user, and geography. Segmenting the market helps in understanding specific market dynamics, targeting customer needs, and identifying growth opportunities within each segment.
Category-wise Insights
Insights into specific categories of Alexipharmic drugs, such as organophosphate antidotes, heavy metal chelators, or cyanide antidotes, provide a deeper understanding of market trends, developments, and challenges related to each category. This information is valuable for industry participants to identify niche areas and tailor their strategies accordingly.
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Strengths:
Critical Antidote Role: Essential in treating poisonings and overdoses with proven clinical efficacy.
Regulatory Support: Fast-track approvals in emergency-use scenarios bolster adoption.
Robust R&D Pipeline: Ongoing research into broad-spectrum antitoxins enhances product portfolios.
Weaknesses:
Niche Demand Base: Usage is limited to poisoning cases, resulting in irregular demand patterns.
High Development Costs: Complex toxicology studies and safety trials increase time and expense.
Storage & Shelf-Life Issues: Many antidotes require cold chain logistics and have limited on-shelf stability.
Opportunities:
Emerging Toxins: New chemical threats (industrial, agricultural) drive need for novel antidotes.
Military & Defense Contracts: Government funding for counter-bioterrorism antidotes.
Combination Formulations: Multi-antidote products could simplify emergency protocols.
Threats:
Alternative Treatments: Supportive care advancements and generic antidotes may reduce demand.
Liability Risks: High legal exposure in cases of treatment failure.
Regulatory Variability: Different standards across countries complicate global launches.
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had an indirect impact on the Alexipharmic drugs market. While not directly related to the virus, the pandemic has affected healthcare systems, supply chains, and emergency response capacities, which can indirectly impact the availability and delivery of antidotes for poisoning cases.
Key Industry Developments
The industry is witnessing various developments such as collaborations between pharmaceutical companies and research institutions, advancements in drug delivery systems, and the emergence of novel antidotes. These developments contribute to the growth and evolution of the Alexipharmic drugs market.
Analyst Suggestions
Based on market trends and insights, analysts suggest the following:
Future Outlook
The future of the Alexipharmic drugs market is promising, driven by advancements in drug development, increased awareness, and collaborations among stakeholders. The market is expected to witness continued growth, with a focus on personalized medicine, technological innovations, and improved patient outcomes.
Conclusion
The Alexipharmic drugs market plays a critical role in treating poisonings and toxicological emergencies. The market is driven by the increasing incidence of poisonings, advancements in drug development and technology, and supportive government initiatives. While there are challenges such as high treatment costs and limited availability of specialized antidotes, opportunities lie in collaborations, emerging markets, product innovation, and personalized medicine approaches. The market is expected to expand, improving patient outcomes and making a positive impact on public health and safety.
What is Alexipharmic Drugs?
Alexipharmic drugs refer to substances that are used to counteract or neutralize the effects of poisons or toxins. These drugs play a crucial role in toxicology and emergency medicine, providing essential treatment options for various types of poisoning.
What are the key players in the Alexipharmic Drugs Market?
Key players in the Alexipharmic Drugs Market include companies such as Pfizer, Merck & Co., and Johnson & Johnson, which are known for their contributions to pharmaceuticals and antidotes, among others.
What are the growth factors driving the Alexipharmic Drugs Market?
The growth of the Alexipharmic Drugs Market is driven by increasing incidences of poisoning, advancements in drug formulations, and a growing awareness of toxicology in healthcare settings. Additionally, the rise in emergency medical services enhances the demand for these drugs.
What challenges does the Alexipharmic Drugs Market face?
The Alexipharmic Drugs Market faces challenges such as regulatory hurdles, the complexity of developing effective antidotes, and the need for extensive clinical trials. Moreover, the variability in poisoning cases can complicate treatment protocols.
What opportunities exist in the Alexipharmic Drugs Market?
Opportunities in the Alexipharmic Drugs Market include the development of novel antidotes for emerging toxins, increased investment in research and development, and potential collaborations between pharmaceutical companies and healthcare providers to improve treatment outcomes.
What trends are shaping the Alexipharmic Drugs Market?
Trends in the Alexipharmic Drugs Market include the integration of technology in drug development, a focus on personalized medicine, and the increasing use of biopharmaceuticals. Additionally, there is a growing emphasis on education and training for healthcare professionals in toxicology.
Alexipharmic Drugs Market:
| Segmentation Details | Description |
|---|---|
| By Drug Class | Activated Charcoal, Chelating Agents, Antidotes, Others |
| By Route of Administration | Oral, Parenteral |
| By Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East and Africa |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Alexipharmic Drugs market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at