444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2450
The Germany continuous glucose monitoring market represents a rapidly evolving segment of the country’s healthcare technology landscape, driven by increasing diabetes prevalence and growing awareness of advanced glucose management solutions. Germany’s healthcare system has embraced continuous glucose monitoring (CGM) technology as a critical component in diabetes care, with adoption rates showing remarkable growth of 12.5% annually across various patient demographics. The market encompasses sophisticated medical devices that provide real-time glucose readings, enabling patients and healthcare providers to make informed decisions about diabetes management.
Healthcare digitization in Germany has accelerated the integration of CGM systems into routine diabetes care, with 65% of endocrinology practices now incorporating these technologies into their treatment protocols. The German market benefits from robust healthcare infrastructure, comprehensive insurance coverage, and strong regulatory frameworks that support innovative medical technologies. Patient outcomes have improved significantly with CGM adoption, showing enhanced glycemic control in 78% of users compared to traditional glucose monitoring methods.
Technological advancement continues to drive market expansion, with next-generation CGM devices offering improved accuracy, longer wear times, and enhanced connectivity features. The integration of artificial intelligence and machine learning algorithms has further enhanced the predictive capabilities of these systems, making them increasingly valuable for both patients and healthcare providers in managing diabetes effectively.
The continuous glucose monitoring market refers to the comprehensive ecosystem of medical devices, software platforms, and healthcare services that enable real-time tracking and analysis of blood glucose levels in diabetic patients. CGM systems utilize small sensors inserted under the skin to measure glucose levels in interstitial fluid, providing continuous data streams that replace or supplement traditional fingerstick blood glucose testing methods.
Market participants include medical device manufacturers, healthcare providers, insurance companies, and technology developers who collectively contribute to the development, distribution, and implementation of CGM solutions. The German market specifically encompasses both prescription-based medical devices and over-the-counter glucose monitoring solutions, serving patients with Type 1 diabetes, Type 2 diabetes, and gestational diabetes.
Healthcare integration within this market involves seamless connectivity between CGM devices, smartphone applications, healthcare provider platforms, and electronic health records systems. This interconnected ecosystem enables comprehensive diabetes management through data sharing, trend analysis, and collaborative care approaches that improve patient outcomes while reducing healthcare costs.
Germany’s continuous glucose monitoring market demonstrates exceptional growth momentum, driven by increasing diabetes prevalence, technological innovation, and supportive healthcare policies. The market has experienced substantial expansion with adoption rates increasing by 15.2% over the past two years, reflecting growing acceptance among both patients and healthcare providers. Key market drivers include aging demographics, rising obesity rates, and enhanced awareness of diabetes complications.
Technology evolution has transformed the CGM landscape, with modern devices offering improved accuracy, extended wear times, and sophisticated data analytics capabilities. Patient satisfaction rates have reached 85% among CGM users, indicating strong market acceptance and potential for continued growth. The integration of digital health platforms and telemedicine services has further enhanced the value proposition of CGM systems.
Market segmentation reveals diverse applications across different diabetes types, with Type 1 diabetes patients representing the largest user base, followed by insulin-dependent Type 2 diabetes patients. Healthcare reimbursement policies in Germany have evolved to support CGM adoption, with coverage rates improving by 22% over recent years, making these technologies more accessible to broader patient populations.
Market dynamics in Germany’s CGM sector reveal several critical insights that shape industry development and future opportunities:
Clinical evidence supporting CGM effectiveness continues to strengthen, with studies demonstrating improved glycemic control, reduced hypoglycemic episodes, and enhanced quality of life for users. Healthcare provider adoption has accelerated as training programs and clinical support systems have matured, creating a more supportive environment for CGM implementation.
Diabetes prevalence represents the primary driver of Germany’s CGM market growth, with epidemiological data indicating steady increases in both Type 1 and Type 2 diabetes diagnoses. Demographic trends show an aging population with higher diabetes risk factors, creating sustained demand for advanced glucose monitoring solutions. The correlation between age-related diabetes complications and the need for precise glucose management has made CGM systems increasingly essential in clinical practice.
Technological advancement continues to drive market expansion through improved device accuracy, user-friendly interfaces, and enhanced connectivity features. Sensor technology improvements have extended wear times and reduced calibration requirements, making CGM systems more convenient and reliable for daily use. Integration with smartphone applications and cloud-based platforms has transformed glucose monitoring from a reactive to a proactive healthcare approach.
Healthcare policy support has emerged as a significant market driver, with German health authorities recognizing the clinical and economic benefits of CGM technology. Reimbursement expansion has made these devices accessible to broader patient populations, while clinical guidelines increasingly recommend CGM use for specific patient groups. The emphasis on preventive healthcare and chronic disease management has further strengthened policy support for CGM adoption.
Patient empowerment trends have created demand for self-monitoring technologies that provide real-time health insights. Digital health literacy improvements among patients have facilitated CGM adoption, while growing awareness of diabetes complications has motivated proactive glucose management approaches.
Cost considerations remain a significant restraint in the German CGM market, particularly for patients with limited insurance coverage or those requiring out-of-pocket payments. Device expenses include not only initial equipment costs but also ongoing sensor replacement requirements, creating financial barriers for some patient populations. Despite improving reimbursement policies, coverage gaps still exist for certain patient categories and device types.
Technical limitations of current CGM systems present ongoing challenges, including sensor accuracy variations, calibration requirements, and occasional device malfunctions. User experience issues such as skin irritation, sensor adhesion problems, and data interpretation difficulties can impact patient compliance and satisfaction. The learning curve associated with CGM technology adoption may discourage some patients from consistent use.
Healthcare provider readiness varies across different medical practices, with some providers lacking adequate training or resources to effectively support CGM implementation. Clinical workflow integration challenges can limit the effectiveness of CGM data utilization in treatment decisions. The need for ongoing patient education and technical support creates additional resource requirements for healthcare providers.
Regulatory complexity surrounding medical device approvals and clinical validation requirements can slow the introduction of innovative CGM technologies. Data privacy concerns related to continuous health monitoring and digital health platforms may create hesitation among some patients and providers regarding CGM adoption.
Artificial intelligence integration presents substantial opportunities for CGM market expansion, with machine learning algorithms offering predictive analytics and personalized treatment recommendations. AI-powered platforms can analyze glucose patterns, predict hypoglycemic episodes, and optimize insulin dosing strategies, significantly enhancing the clinical value of CGM systems. The development of intelligent alert systems and automated treatment adjustments represents a major growth opportunity.
Telemedicine expansion creates new opportunities for remote diabetes management using CGM data, particularly relevant in post-pandemic healthcare delivery models. Remote monitoring capabilities enable healthcare providers to track patient glucose patterns continuously, facilitating timely interventions and reducing the need for frequent office visits. This approach improves healthcare accessibility while maintaining quality care standards.
Pediatric market development offers significant growth potential, with increasing recognition of CGM benefits for children with diabetes. Family-centered care approaches using CGM technology can improve diabetes management in pediatric populations while reducing caregiver anxiety. Specialized pediatric CGM devices and educational programs represent emerging market opportunities.
Integration with wearable technology and Internet of Things (IoT) devices creates opportunities for comprehensive health monitoring ecosystems. Smartwatch compatibility and fitness tracker integration can enhance user engagement while providing additional health insights that complement glucose monitoring data.
Competitive dynamics in Germany’s CGM market reflect a balance between established medical device manufacturers and innovative technology companies entering the diabetes care space. Market consolidation trends have emerged as larger companies acquire specialized CGM developers to enhance their product portfolios and market reach. This consolidation has accelerated innovation while creating more comprehensive diabetes management solutions.
Supply chain evolution has adapted to support the unique requirements of CGM distribution, including cold chain management, sensor shelf-life considerations, and direct-to-patient delivery models. Distribution partnerships between manufacturers and healthcare providers have streamlined CGM access while ensuring appropriate clinical support for users. The development of specialized diabetes care centers has created new distribution channels for CGM products.
Regulatory dynamics continue to evolve as authorities balance innovation encouragement with patient safety requirements. Approval processes have become more streamlined for CGM devices, while post-market surveillance requirements have intensified to ensure ongoing safety and effectiveness. The harmonization of European medical device regulations has facilitated market access for international CGM manufacturers.
Healthcare economics play an increasingly important role in CGM adoption decisions, with cost-effectiveness analyses demonstrating long-term healthcare savings through improved diabetes management. Value-based care models are beginning to incorporate CGM utilization as quality metrics, creating additional incentives for adoption.
Market research methodology for the German CGM market employs comprehensive primary and secondary research approaches to ensure accurate and reliable market intelligence. Primary research includes structured interviews with healthcare providers, patient surveys, and expert consultations with endocrinologists and diabetes educators across Germany. This direct engagement provides insights into real-world CGM utilization patterns, clinical outcomes, and user satisfaction levels.
Secondary research encompasses analysis of published clinical studies, regulatory filings, company financial reports, and healthcare policy documents. Data triangulation methods validate findings across multiple sources to ensure accuracy and reliability. Market sizing and forecasting utilize statistical modeling techniques that account for demographic trends, healthcare policy changes, and technology adoption patterns.
Quantitative analysis incorporates epidemiological data, healthcare utilization statistics, and device sales information to establish market baselines and growth projections. Qualitative research explores market dynamics, competitive positioning, and emerging trends through expert interviews and focus group discussions. The methodology ensures comprehensive coverage of market segments, geographic regions, and stakeholder perspectives.
Data validation processes include cross-referencing multiple sources, expert review panels, and statistical significance testing to ensure research reliability. Continuous monitoring of market developments enables real-time updates to research findings and market projections.
Geographic distribution of CGM adoption across Germany reveals distinct regional patterns influenced by healthcare infrastructure, population demographics, and provider availability. Urban centers including Berlin, Munich, Hamburg, and Frankfurt demonstrate higher CGM penetration rates, with metropolitan areas accounting for 58% of total market adoption. These regions benefit from concentrated endocrinology practices, advanced healthcare facilities, and higher digital health literacy among populations.
Northern Germany shows strong CGM adoption driven by comprehensive healthcare coverage and proactive diabetes management programs. Healthcare networks in this region have implemented systematic CGM integration protocols, resulting in adoption rates 18% above national averages. The presence of major medical centers and research institutions has facilitated early technology adoption and clinical validation studies.
Southern Germany demonstrates robust market growth supported by strong healthcare infrastructure and higher disposable incomes that facilitate technology adoption. Bavaria and Baden-Württemberg lead regional CGM utilization, with specialized diabetes centers driving clinical adoption and patient education initiatives. The region’s focus on medical technology innovation has attracted CGM manufacturers and research collaborations.
Eastern Germany presents emerging opportunities as healthcare modernization efforts expand access to advanced medical technologies. Infrastructure development and targeted healthcare investment programs are gradually improving CGM accessibility in these regions, with growth rates exceeding 20% annually as adoption barriers are addressed.
Market leadership in Germany’s CGM sector is characterized by intense competition among established medical device manufacturers and innovative technology companies. The competitive environment drives continuous innovation while ensuring diverse product options for patients and healthcare providers.
Competitive strategies focus on technological differentiation, clinical evidence generation, and healthcare provider partnerships. Product innovation remains the primary competitive differentiator, with companies investing heavily in sensor accuracy improvements, extended wear times, and enhanced connectivity features. Strategic partnerships with healthcare systems and insurance providers have become increasingly important for market access and adoption.
Market positioning varies among competitors, with some focusing on premium technology solutions while others emphasize cost-effectiveness and accessibility. Clinical validation through peer-reviewed studies and real-world evidence generation has become essential for competitive success in the German market.
Market segmentation of Germany’s CGM market reveals distinct categories based on technology type, patient demographics, and clinical applications. Technology-based segmentation includes real-time CGM systems, flash glucose monitoring devices, and emerging implantable sensors, each serving different patient needs and clinical scenarios.
By Technology:
By Patient Type:
By End User:
Real-time CGM systems dominate the German market due to their comprehensive monitoring capabilities and clinical validation in diabetes management. Healthcare providers prefer these systems for patients requiring intensive glucose management, particularly those with Type 1 diabetes or unstable Type 2 diabetes. The ability to provide continuous glucose trends and predictive alerts makes real-time CGM systems essential for preventing dangerous glucose excursions.
Flash glucose monitoring has gained significant traction among patients seeking convenient glucose monitoring without the complexity of traditional CGM systems. User adoption has been particularly strong among Type 2 diabetes patients who appreciate the simplicity and lower cost of flash monitoring technology. The elimination of routine fingerstick calibrations has improved patient compliance and satisfaction.
Pediatric CGM applications represent a rapidly growing category, with specialized devices designed for children’s unique needs including smaller sensors, colorful designs, and family-sharing capabilities. Caregiver features such as remote monitoring and alert sharing have made CGM systems invaluable tools for managing childhood diabetes while maintaining normal activities and reducing family stress.
Hospital-based CGM utilization has expanded beyond traditional diabetes care to include surgical patients, critical care monitoring, and medication management applications. Clinical integration with electronic health records and clinical decision support systems has enhanced the utility of CGM data in hospital settings.
Healthcare providers benefit from CGM technology through improved patient outcomes, enhanced clinical decision-making capabilities, and more efficient diabetes management workflows. Clinical advantages include access to comprehensive glucose data, trend analysis capabilities, and remote monitoring options that enable proactive patient care. The reduction in emergency department visits and diabetes-related complications creates operational efficiencies and cost savings for healthcare systems.
Patients experience significant quality of life improvements through CGM adoption, including reduced fingerstick testing requirements, better glucose control, and decreased anxiety about hypoglycemic episodes. Lifestyle benefits encompass greater flexibility in daily activities, improved sleep quality, and enhanced confidence in diabetes self-management. The educational value of continuous glucose data helps patients understand the impact of food, exercise, and medication on their glucose levels.
Insurance providers realize long-term cost benefits through reduced diabetes complications, fewer emergency interventions, and improved chronic disease management outcomes. Economic advantages include decreased hospitalization rates, reduced specialist consultations, and lower overall healthcare utilization among CGM users. The preventive nature of CGM technology aligns with value-based care models and population health management strategies.
Technology manufacturers benefit from growing market demand, opportunities for innovation, and expanding applications beyond traditional diabetes care. Business advantages include recurring revenue from sensor replacements, data analytics opportunities, and potential integration with broader digital health platforms.
Strengths:
Weaknesses:
Opportunities:
Threats:
Digital health integration represents the most significant trend shaping Germany’s CGM market, with devices increasingly connecting to smartphone applications, cloud platforms, and healthcare provider systems. Data analytics capabilities have evolved beyond simple glucose tracking to include predictive modeling, pattern recognition, and personalized treatment recommendations. This integration enables more sophisticated diabetes management approaches and improved patient outcomes.
Artificial intelligence adoption is transforming CGM functionality through machine learning algorithms that can predict glucose trends, identify patterns, and provide automated insights. AI-powered features include hypoglycemia prediction, insulin dosing recommendations, and lifestyle impact analysis. According to MarkWide Research analysis, AI integration is expected to become standard in next-generation CGM systems.
Miniaturization trends continue to drive CGM device development, with manufacturers focusing on smaller sensors, longer wear times, and improved comfort. User experience enhancements include waterproof designs, flexible sensors, and reduced skin irritation. The trend toward discrete, comfortable devices has improved patient acceptance and compliance rates.
Interoperability standards are emerging to enable seamless data sharing between different CGM systems, healthcare providers, and digital health platforms. Standardization efforts facilitate better care coordination and reduce technology barriers in clinical practice.
Regulatory approvals for next-generation CGM devices have accelerated market innovation, with German and European authorities streamlining approval processes for devices demonstrating clear clinical benefits. Recent approvals include extended-wear sensors, implantable CGM systems, and integrated insulin delivery platforms that combine glucose monitoring with automated insulin administration.
Clinical evidence supporting CGM effectiveness continues to strengthen through large-scale studies and real-world evidence collection. Research findings demonstrate improved glycemic control, reduced hypoglycemic episodes, and enhanced quality of life among CGM users across different patient populations. These studies have supported expanded reimbursement coverage and clinical guideline updates.
Technology partnerships between CGM manufacturers and digital health companies have created comprehensive diabetes management platforms. Strategic alliances focus on data analytics, artificial intelligence integration, and telemedicine capabilities that enhance the clinical value of CGM systems. These partnerships have accelerated innovation while expanding market reach.
Healthcare policy developments have increasingly recognized CGM technology as essential diabetes care tools, leading to expanded coverage criteria and reduced access barriers. Policy changes include streamlined prescription processes, expanded patient eligibility criteria, and improved reimbursement rates that support broader CGM adoption.
Market expansion strategies should focus on addressing remaining access barriers through innovative financing models, patient assistance programs, and value-based care partnerships. Manufacturers should prioritize development of cost-effective CGM solutions that maintain clinical effectiveness while improving affordability. The creation of tiered product offerings can serve diverse patient needs and economic circumstances.
Healthcare provider education remains critical for successful CGM implementation, requiring comprehensive training programs, clinical decision support tools, and ongoing technical assistance. Educational initiatives should emphasize practical CGM integration strategies, data interpretation skills, and patient counseling techniques. Partnership with medical societies and continuing education providers can enhance training reach and effectiveness.
Patient engagement strategies should leverage digital health platforms, peer support networks, and gamification approaches to improve CGM utilization and outcomes. Support programs should address common barriers including technology anxiety, data interpretation challenges, and lifestyle integration difficulties. Personalized education and ongoing support can significantly improve patient success with CGM technology.
Innovation priorities should focus on accuracy improvements, extended wear times, and enhanced predictive capabilities that provide greater clinical value. Research investments in artificial intelligence, sensor technology, and user interface design can drive competitive differentiation and market growth. Collaboration with academic institutions and clinical researchers can accelerate innovation timelines.
Market growth projections for Germany’s CGM sector indicate continued expansion driven by demographic trends, technology advancement, and healthcare policy support. Adoption rates are expected to increase significantly, with penetration reaching 45% of eligible patients within the next five years. The expansion will be supported by improved reimbursement coverage, enhanced clinical evidence, and growing provider acceptance.
Technology evolution will transform CGM capabilities through artificial intelligence integration, improved sensor accuracy, and seamless healthcare system connectivity. Next-generation devices will offer predictive analytics, automated treatment recommendations, and integrated diabetes management platforms that provide comprehensive care solutions. MWR projects that AI-enabled CGM systems will represent over 60% of new device sales by 2028.
Healthcare integration will deepen as CGM data becomes standard in electronic health records, clinical decision support systems, and population health management programs. Interoperability standards will enable seamless data sharing across healthcare providers, improving care coordination and patient outcomes. The integration of CGM data with other health metrics will create comprehensive patient health profiles.
Market expansion will extend beyond traditional diabetes care to include pre-diabetes monitoring, gestational diabetes management, and metabolic health optimization applications. Emerging applications in sports medicine, wellness monitoring, and chronic disease prevention will create new market opportunities and user segments.
Germany’s continuous glucose monitoring market stands at a pivotal point of growth and transformation, driven by compelling clinical evidence, supportive healthcare policies, and continuous technological innovation. The market has demonstrated remarkable resilience and expansion potential, with adoption rates accelerating across diverse patient populations and healthcare settings. The convergence of demographic trends, technology advancement, and healthcare digitization creates a favorable environment for sustained market growth.
Key success factors for market participants include focus on clinical value demonstration, patient education and support, healthcare provider partnerships, and continuous innovation in device capabilities and user experience. The integration of artificial intelligence, telemedicine platforms, and comprehensive diabetes management solutions will define competitive advantage in the evolving market landscape. Stakeholder collaboration between manufacturers, healthcare providers, payers, and patients will be essential for maximizing the clinical and economic benefits of CGM technology.
Future market development will be characterized by expanded applications, improved accessibility, and enhanced integration with broader healthcare systems. The German CGM market is well-positioned to lead European adoption trends while serving as a model for comprehensive diabetes care integration. Continued investment in research, education, and infrastructure development will ensure that Germany remains at the forefront of CGM innovation and implementation, ultimately improving outcomes for the growing population of individuals living with diabetes.
What is Continuous Glucose Monitoring?
Continuous Glucose Monitoring refers to a method of tracking glucose levels in real-time using a small sensor placed under the skin. This technology is primarily used by individuals with diabetes to manage their blood sugar levels effectively.
What are the key players in the Germany Continuous Glucose Monitoring Market?
Key players in the Germany Continuous Glucose Monitoring Market include Dexcom, Abbott Laboratories, Medtronic, and Roche, among others. These companies are known for their innovative products and technologies in glucose monitoring.
What are the growth factors driving the Germany Continuous Glucose Monitoring Market?
The growth of the Germany Continuous Glucose Monitoring Market is driven by the increasing prevalence of diabetes, advancements in sensor technology, and the rising demand for personalized healthcare solutions. Additionally, the growing awareness of diabetes management among patients contributes to market expansion.
What challenges does the Germany Continuous Glucose Monitoring Market face?
The Germany Continuous Glucose Monitoring Market faces challenges such as high costs of devices, regulatory hurdles, and the need for continuous calibration. These factors can limit accessibility and adoption among patients.
What opportunities exist in the Germany Continuous Glucose Monitoring Market?
Opportunities in the Germany Continuous Glucose Monitoring Market include the development of more affordable devices, integration with digital health platforms, and the potential for expanded use in non-diabetic populations for preventive health monitoring. These trends can enhance market growth.
What trends are shaping the Germany Continuous Glucose Monitoring Market?
Trends shaping the Germany Continuous Glucose Monitoring Market include the rise of smartphone connectivity for data sharing, the development of non-invasive monitoring technologies, and an increasing focus on patient-centric care. These innovations are transforming how glucose monitoring is approached.
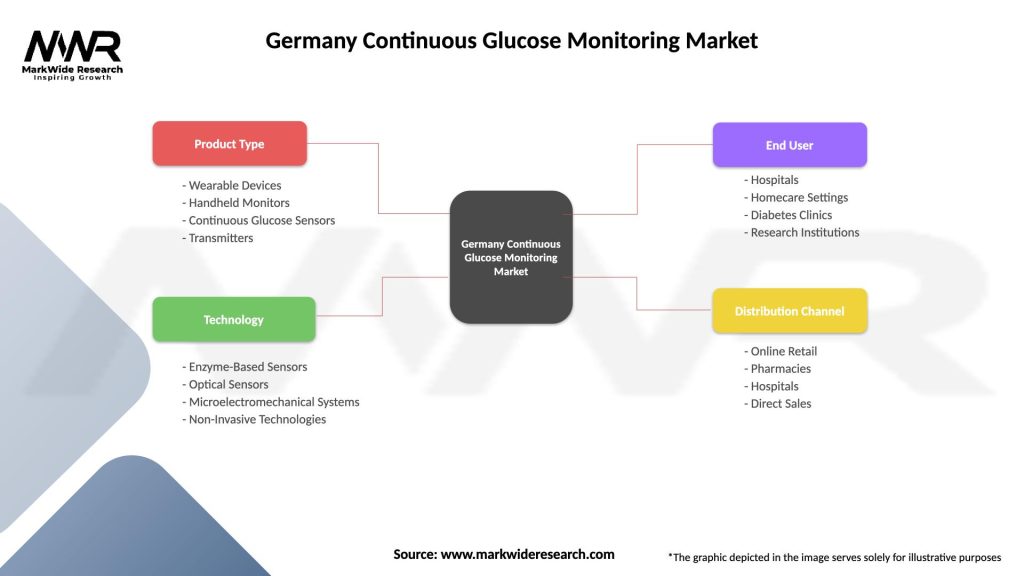
Germany Continuous Glucose Monitoring Market
| Segmentation Details | Description |
|---|---|
| Product Type | Wearable Devices, Handheld Monitors, Continuous Glucose Sensors, Transmitters |
| Technology | Enzyme-Based Sensors, Optical Sensors, Microelectromechanical Systems, Non-Invasive Technologies |
| End User | Hospitals, Homecare Settings, Diabetes Clinics, Research Institutions |
| Distribution Channel | Online Retail, Pharmacies, Hospitals, Direct Sales |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Germany Continuous Glucose Monitoring Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at