444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
The Asia Pacific cath lab hemodynamic monitoring systems market represents one of the most rapidly evolving segments within the region’s healthcare technology landscape. This dynamic market encompasses sophisticated monitoring equipment designed to measure and analyze cardiovascular parameters during cardiac catheterization procedures. Regional healthcare systems are experiencing unprecedented demand for advanced hemodynamic monitoring solutions as cardiovascular disease prevalence continues to rise across Asia Pacific nations.
Market expansion is being driven by increasing investments in cardiac care infrastructure, particularly in emerging economies such as India, China, and Southeast Asian countries. The region’s aging population, coupled with lifestyle-related cardiovascular risk factors, has created substantial demand for comprehensive cardiac monitoring capabilities. Healthcare providers are increasingly adopting integrated hemodynamic monitoring systems that offer real-time data analysis, enhanced patient safety protocols, and improved procedural outcomes.
Technological advancement in the Asia Pacific region is characterized by the integration of artificial intelligence, wireless connectivity, and cloud-based data management systems. These innovations are transforming traditional cath lab operations, enabling healthcare professionals to make more informed decisions during critical cardiac interventions. The market is projected to experience robust growth at a CAGR of 8.2% over the forecast period, reflecting the region’s commitment to modernizing cardiovascular care infrastructure.
The Asia Pacific cath lab hemodynamic monitoring systems market refers to the comprehensive ecosystem of medical devices, software solutions, and integrated platforms designed to monitor cardiovascular parameters during cardiac catheterization procedures across the Asia Pacific region. These sophisticated systems measure critical hemodynamic variables including blood pressure, cardiac output, pulmonary pressures, and vascular resistance in real-time during interventional cardiology procedures.
Hemodynamic monitoring systems encompass a range of technologies from basic pressure monitoring devices to advanced multi-parameter platforms that integrate with hospital information systems. These solutions enable cardiologists and cardiac surgeons to assess cardiovascular function, guide therapeutic interventions, and optimize patient outcomes during complex cardiac procedures. The market includes both invasive and non-invasive monitoring technologies, each serving specific clinical applications within modern cath lab environments.
Regional market dynamics are influenced by varying healthcare infrastructure development levels, regulatory frameworks, and economic conditions across different Asia Pacific countries. The market encompasses established healthcare systems in Japan, Australia, and South Korea, alongside rapidly developing markets in China, India, and Southeast Asia, each presenting unique opportunities and challenges for hemodynamic monitoring system adoption.
Strategic market analysis reveals that the Asia Pacific cath lab hemodynamic monitoring systems market is positioned for substantial expansion driven by demographic shifts, technological innovation, and healthcare infrastructure investments. The region’s cardiovascular disease burden continues to escalate, with cardiovascular conditions accounting for approximately 35% of all deaths across major Asia Pacific countries, creating urgent demand for advanced monitoring capabilities.
Market segmentation demonstrates strong growth across multiple categories, with invasive hemodynamic monitoring systems maintaining dominant market share while non-invasive alternatives gain traction in cost-sensitive markets. The integration of digital health technologies, including telemedicine capabilities and remote monitoring features, is reshaping traditional cath lab operations and expanding market opportunities beyond conventional hospital settings.
Competitive dynamics are characterized by intense innovation cycles, strategic partnerships between global medical device manufacturers and regional healthcare providers, and increasing emphasis on value-based healthcare solutions. Market leaders are focusing on developing comprehensive platforms that combine monitoring capabilities with data analytics, artificial intelligence, and predictive modeling to enhance clinical decision-making and patient outcomes.
Regional growth patterns show China and India leading market expansion, collectively representing over 60% of regional demand, while developed markets like Japan and Australia drive premium technology adoption. The market’s future trajectory is closely linked to healthcare digitization initiatives, regulatory harmonization efforts, and continued investment in cardiac care infrastructure across the region.
Critical market insights reveal several transformative trends shaping the Asia Pacific cath lab hemodynamic monitoring systems landscape. The following key observations provide strategic understanding of market dynamics:
Primary market drivers propelling the Asia Pacific cath lab hemodynamic monitoring systems market include demographic, technological, and healthcare policy factors that create sustained demand for advanced cardiovascular monitoring solutions. The region’s rapidly aging population represents a fundamental driver, with individuals over 65 years experiencing significantly higher rates of cardiovascular disease requiring interventional procedures.
Healthcare infrastructure development across emerging Asia Pacific economies is creating substantial market opportunities. Government initiatives to modernize hospital systems, establish cardiac care centers, and improve healthcare accessibility are driving demand for sophisticated monitoring equipment. Countries like India and China are investing heavily in healthcare infrastructure, with cardiac care facilities representing priority areas for technology adoption.
Rising cardiovascular disease prevalence across the region is creating urgent demand for advanced monitoring capabilities. Lifestyle changes, urbanization, and dietary shifts have contributed to increasing rates of coronary artery disease, heart failure, and other cardiovascular conditions requiring interventional treatment. This epidemiological trend is expected to continue, sustaining long-term market growth.
Technological advancement in medical device manufacturing is enabling the development of more sophisticated, user-friendly, and cost-effective hemodynamic monitoring solutions. Integration of digital technologies, including artificial intelligence, machine learning, and advanced data analytics, is enhancing the clinical value proposition of modern monitoring systems and driving healthcare provider adoption.
Significant market restraints present challenges to the widespread adoption of advanced hemodynamic monitoring systems across the Asia Pacific region. High capital investment requirements represent a primary barrier, particularly for healthcare facilities in emerging markets where budget constraints limit technology acquisition capabilities. The substantial costs associated with system implementation, staff training, and ongoing maintenance can deter smaller healthcare providers from adopting advanced monitoring solutions.
Regulatory complexity across different Asia Pacific countries creates market entry challenges for medical device manufacturers. Varying approval processes, clinical trial requirements, and quality standards necessitate significant resources and time investments to achieve market access. These regulatory hurdles can delay product launches and increase development costs, particularly for innovative technologies requiring extensive validation.
Healthcare professional training requirements present operational challenges for healthcare facilities implementing new monitoring systems. The sophisticated nature of modern hemodynamic monitoring equipment requires specialized training programs, which can be resource-intensive and time-consuming. Limited availability of trained personnel in some regions can constrain market growth and technology adoption rates.
Infrastructure limitations in certain Asia Pacific markets, including inadequate power supply, limited internet connectivity, and insufficient technical support networks, can hinder the deployment of advanced monitoring systems. These infrastructure challenges are particularly pronounced in rural areas and developing economies, limiting market penetration in underserved regions.
Substantial market opportunities are emerging across the Asia Pacific cath lab hemodynamic monitoring systems market, driven by technological innovation, healthcare digitization, and expanding access to cardiac care services. The integration of telemedicine capabilities with hemodynamic monitoring systems presents significant opportunities for remote patient monitoring and consultation services, particularly relevant in the post-pandemic healthcare environment.
Artificial intelligence integration represents a transformative opportunity for market expansion. Advanced AI algorithms can enhance diagnostic accuracy, predict patient complications, and optimize treatment protocols, creating substantial value for healthcare providers. Companies developing AI-powered hemodynamic monitoring solutions are positioned to capture significant market share as healthcare systems prioritize data-driven decision-making.
Emerging market penetration offers substantial growth opportunities, particularly in Southeast Asian countries, where healthcare infrastructure development is accelerating. Government initiatives to improve cardiac care access, combined with increasing healthcare spending, create favorable conditions for market expansion. Strategic partnerships with local healthcare providers and distributors can facilitate market entry and growth in these regions.
Value-based healthcare models are creating opportunities for comprehensive monitoring solutions that demonstrate improved patient outcomes and cost-effectiveness. Healthcare providers are increasingly interested in systems that can reduce procedure times, minimize complications, and enhance overall care quality, creating market demand for integrated monitoring platforms with proven clinical benefits.
Complex market dynamics shape the Asia Pacific cath lab hemodynamic monitoring systems landscape, reflecting the interplay between technological advancement, healthcare policy changes, and evolving clinical practices. The market is experiencing a fundamental shift toward integrated, digitally-enabled monitoring solutions that provide comprehensive cardiovascular assessment capabilities within modern cath lab environments.
Competitive intensity is increasing as global medical device manufacturers expand their Asia Pacific presence while regional companies develop innovative, cost-effective solutions tailored to local market requirements. This competitive dynamic is driving rapid innovation cycles, with companies investing heavily in research and development to differentiate their offerings and capture market share.
Healthcare digitization trends are transforming traditional monitoring approaches, with healthcare providers increasingly demanding systems that integrate seamlessly with electronic health records, hospital information systems, and telemedicine platforms. This digital transformation is creating new market segments and business models, including software-as-a-service offerings and cloud-based monitoring solutions.
Regulatory evolution across Asia Pacific countries is gradually harmonizing standards and approval processes, facilitating market access for innovative monitoring technologies. However, regional variations in regulatory requirements continue to influence market entry strategies and product development priorities for medical device manufacturers operating in multiple countries.
Comprehensive research methodology employed in analyzing the Asia Pacific cath lab hemodynamic monitoring systems market incorporates multiple data sources, analytical frameworks, and validation techniques to ensure accuracy and reliability of market insights. The research approach combines quantitative analysis of market trends with qualitative assessment of industry dynamics, competitive positioning, and technological developments.
Primary research activities include extensive interviews with healthcare professionals, hospital administrators, medical device manufacturers, and industry experts across key Asia Pacific markets. These interviews provide firsthand insights into market trends, technology adoption patterns, and future growth prospects. Survey data from healthcare facilities regarding their monitoring system requirements and investment priorities inform market sizing and segmentation analysis.
Secondary research sources encompass industry reports, government healthcare statistics, medical device regulatory databases, and academic publications related to cardiovascular monitoring technologies. Analysis of patent filings, clinical trial data, and regulatory approvals provides insights into technological innovation trends and competitive dynamics within the market.
Data validation processes include cross-referencing multiple sources, conducting expert interviews to verify findings, and applying statistical analysis techniques to ensure data consistency and reliability. Market projections are developed using econometric modeling approaches that account for regional economic conditions, healthcare spending trends, and demographic factors influencing market growth.
Regional market analysis reveals significant variations in hemodynamic monitoring system adoption, growth patterns, and market characteristics across different Asia Pacific countries. China represents the largest regional market, accounting for approximately 42% of total demand, driven by massive healthcare infrastructure investments, growing cardiovascular disease prevalence, and government initiatives to modernize hospital systems.
Japan maintains a leadership position in premium technology adoption, with healthcare facilities demonstrating strong preference for advanced, integrated monitoring solutions. The Japanese market is characterized by high technology penetration rates, sophisticated clinical practices, and willingness to invest in innovative medical devices that enhance patient outcomes and procedural efficiency.
India represents the fastest-growing regional market, with demand increasing at an accelerated pace due to expanding healthcare access, rising cardiovascular disease rates, and increasing healthcare spending. The Indian market shows strong preference for cost-effective monitoring solutions that provide essential functionality while maintaining affordability for diverse healthcare settings.
Southeast Asian markets, including Thailand, Malaysia, Singapore, and Indonesia, collectively account for approximately 18% of regional demand. These markets are experiencing rapid growth driven by healthcare infrastructure development, medical tourism expansion, and increasing government investment in cardiac care capabilities. Australia and South Korea represent mature markets with stable demand patterns and focus on technology upgrades and system replacements.
Competitive landscape analysis reveals a dynamic market environment characterized by intense competition between global medical device manufacturers and emerging regional players. The market features several distinct competitive segments, each serving different customer requirements and price points across the Asia Pacific region.
Leading market participants include established global companies that have developed comprehensive hemodynamic monitoring platforms with advanced features and proven clinical efficacy:
Regional competitors are gaining market share by developing cost-effective solutions tailored to local market requirements, offering competitive pricing, and providing comprehensive local support services. These companies often focus on specific market segments or geographic regions where they can leverage local relationships and market knowledge.
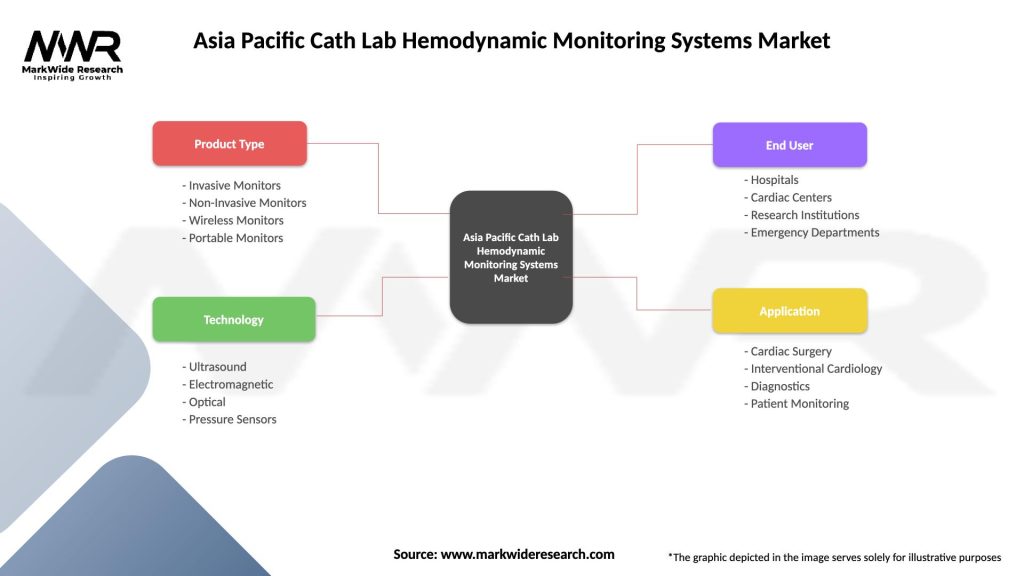
Market segmentation analysis provides detailed insights into different categories within the Asia Pacific cath lab hemodynamic monitoring systems market, revealing distinct growth patterns, customer preferences, and competitive dynamics across various segments. The market can be analyzed across multiple dimensions including technology type, application area, end-user category, and geographic region.
By Technology Type:
By Application Area:
By End User:
Invasive hemodynamic monitoring systems continue to dominate the Asia Pacific market, representing approximately 68% of total segment share due to their established clinical efficacy and widespread acceptance among cardiovascular specialists. These systems provide direct measurement of intracardiac and arterial pressures, offering the highest accuracy for critical clinical decision-making during complex cardiac procedures.
Non-invasive monitoring technologies are experiencing rapid growth, particularly in markets where cost considerations and patient safety concerns drive adoption decisions. These systems utilize advanced algorithms and sensor technologies to estimate hemodynamic parameters without requiring invasive catheter placement, making them attractive for routine monitoring applications and resource-constrained healthcare settings.
Minimally invasive monitoring solutions represent an emerging category that combines the accuracy advantages of invasive monitoring with reduced patient risk and improved comfort. These systems typically require smaller catheter sizes or alternative measurement approaches that minimize procedural complexity while maintaining clinical utility for hemodynamic assessment.
Integrated monitoring platforms are gaining market traction as healthcare providers seek comprehensive solutions that combine hemodynamic monitoring with other cardiac assessment capabilities. These platforms often include electrocardiographic monitoring, imaging integration, and data management features that streamline cath lab workflows and enhance procedural efficiency.
Healthcare providers benefit significantly from advanced hemodynamic monitoring systems through improved patient outcomes, enhanced procedural safety, and optimized clinical workflows. These systems enable real-time assessment of cardiovascular function, allowing clinicians to make informed decisions during critical procedures and adjust treatment strategies based on objective hemodynamic data.
Patients experience substantial benefits including reduced procedure times, minimized complications, and improved treatment outcomes through the use of advanced monitoring technologies. Modern systems enable less invasive procedures while maintaining diagnostic accuracy, resulting in faster recovery times and reduced hospital stays for many patients undergoing cardiac interventions.
Medical device manufacturers gain competitive advantages through the development of innovative monitoring solutions that address evolving clinical needs and market requirements. Companies investing in advanced technologies, including artificial intelligence integration and wireless connectivity, can differentiate their offerings and capture premium market segments.
Healthcare systems benefit from improved resource utilization, reduced complication rates, and enhanced quality metrics through the adoption of sophisticated monitoring technologies. These systems can contribute to value-based healthcare initiatives by demonstrating improved patient outcomes and cost-effectiveness compared to traditional monitoring approaches.
Regulatory bodies benefit from standardized monitoring protocols and improved patient safety data generated by advanced hemodynamic monitoring systems. These technologies can contribute to evidence-based regulatory decisions and support the development of clinical guidelines for cardiovascular care.
Strengths:
Weaknesses:
Opportunities:
Threats:
Digital transformation trends are fundamentally reshaping the Asia Pacific cath lab hemodynamic monitoring systems market, with healthcare providers increasingly demanding integrated solutions that connect seamlessly with hospital information systems, electronic health records, and telemedicine platforms. This digital integration enables comprehensive patient data management, remote consultation capabilities, and enhanced clinical decision support.
Artificial intelligence adoption is accelerating across the region, with monitoring systems incorporating machine learning algorithms for predictive analytics, automated pattern recognition, and clinical decision support. These AI-powered capabilities enable early detection of hemodynamic changes, prediction of potential complications, and optimization of treatment protocols based on real-time patient data analysis.
Miniaturization and portability trends are driving the development of compact, wireless monitoring solutions that reduce patient discomfort while maintaining measurement accuracy. These portable systems enable monitoring in diverse clinical settings, including ambulatory care facilities, emergency departments, and transport situations where traditional monitoring equipment may be impractical.
Value-based healthcare adoption is influencing purchasing decisions, with healthcare providers increasingly evaluating monitoring systems based on their ability to demonstrate improved patient outcomes, reduced complications, and cost-effectiveness. This trend is driving manufacturers to develop comprehensive solutions that provide clear clinical and economic value propositions.
Personalized medicine integration is emerging as monitoring systems incorporate patient-specific algorithms and customizable parameters that account for individual patient characteristics, medical history, and risk factors. This personalization enables more precise hemodynamic assessment and tailored treatment approaches for optimal patient outcomes.
Recent industry developments demonstrate the dynamic nature of the Asia Pacific cath lab hemodynamic monitoring systems market, with significant advancements in technology, regulatory approvals, and strategic partnerships shaping the competitive landscape. MarkWide Research analysis indicates that product innovation cycles are accelerating as companies respond to evolving clinical needs and market opportunities.
Regulatory milestone achievements include several breakthrough device designations and expedited approvals for innovative monitoring technologies across major Asia Pacific markets. These regulatory successes are facilitating faster market entry for advanced monitoring solutions and encouraging continued investment in research and development activities.
Strategic partnerships between global medical device manufacturers and regional healthcare providers are expanding market access and accelerating technology adoption. These collaborations often include comprehensive training programs, technical support services, and customized solutions designed to meet specific regional market requirements.
Technology integration initiatives are advancing rapidly, with companies developing comprehensive platforms that combine hemodynamic monitoring with imaging capabilities, electronic health record integration, and artificial intelligence-powered analytics. These integrated solutions are gaining market acceptance as healthcare providers seek to streamline workflows and enhance clinical efficiency.
Clinical evidence generation continues to support market growth, with numerous clinical studies demonstrating the benefits of advanced hemodynamic monitoring in improving patient outcomes, reducing complications, and optimizing resource utilization. This growing evidence base is supporting adoption decisions and reimbursement approvals across the region.
Strategic recommendations for market participants focus on leveraging emerging opportunities while addressing key challenges in the Asia Pacific cath lab hemodynamic monitoring systems market. Companies should prioritize the development of integrated, digitally-enabled solutions that provide comprehensive monitoring capabilities while addressing regional cost sensitivity and infrastructure limitations.
Technology investment priorities should emphasize artificial intelligence integration, wireless connectivity, and cloud-based data management capabilities that align with healthcare digitization trends. Companies developing AI-powered monitoring solutions with predictive analytics capabilities are positioned to capture significant market share as healthcare providers increasingly adopt data-driven clinical decision-making approaches.
Market entry strategies for emerging Asia Pacific markets should focus on developing cost-effective solutions tailored to local healthcare infrastructure and budget constraints. Strategic partnerships with regional distributors, healthcare providers, and government agencies can facilitate market access and accelerate adoption in underserved markets.
Clinical evidence development remains critical for market success, with companies advised to invest in comprehensive clinical studies that demonstrate the safety, efficacy, and economic benefits of their monitoring solutions. Strong clinical evidence supports regulatory approvals, reimbursement decisions, and healthcare provider adoption across diverse Asia Pacific markets.
Training and support programs should be prioritized to address the technical complexity of advanced monitoring systems and ensure optimal utilization by healthcare professionals. Comprehensive education initiatives can accelerate technology adoption and improve patient outcomes while building long-term customer relationships and market loyalty.
Future market projections indicate sustained growth for the Asia Pacific cath lab hemodynamic monitoring systems market, driven by demographic trends, technological advancement, and healthcare infrastructure development across the region. The market is expected to experience robust expansion with growth rates projected at 8.2% CAGR over the next decade, reflecting strong underlying demand drivers and favorable market conditions.
Technological evolution will continue to reshape the market landscape, with artificial intelligence, machine learning, and advanced data analytics becoming standard features in next-generation monitoring systems. These technologies will enable more sophisticated clinical decision support, predictive modeling, and personalized treatment approaches that enhance patient outcomes and clinical efficiency.
Market expansion into emerging Asia Pacific countries is expected to accelerate as healthcare infrastructure development continues and government investment in cardiac care capabilities increases. Countries such as Vietnam, Philippines, and Bangladesh represent significant growth opportunities as their healthcare systems modernize and expand access to advanced cardiac care services.
Integration trends will drive the development of comprehensive cath lab platforms that combine hemodynamic monitoring with imaging, therapeutic devices, and data management systems. These integrated solutions will become increasingly important as healthcare providers seek to optimize workflows, reduce costs, and improve patient outcomes through coordinated care approaches.
Regulatory harmonization across Asia Pacific countries is expected to facilitate market access and reduce barriers to innovation, enabling faster deployment of advanced monitoring technologies and supporting continued market growth. According to MWR projections, regulatory standardization efforts will contribute to market efficiency and accelerated technology adoption across the region.
The Asia Pacific cath lab hemodynamic monitoring systems market represents a dynamic and rapidly evolving segment of the regional healthcare technology landscape, characterized by strong growth prospects, technological innovation, and expanding clinical applications. The market’s trajectory is supported by fundamental demographic trends, including population aging and increasing cardiovascular disease prevalence, which create sustained demand for advanced monitoring capabilities.
Technological advancement continues to drive market evolution, with artificial intelligence integration, wireless connectivity, and digital health platform development transforming traditional monitoring approaches. These innovations are enhancing clinical value propositions while addressing regional market requirements for cost-effective, user-friendly solutions that improve patient outcomes and procedural efficiency.
Regional market dynamics demonstrate significant opportunities across diverse Asia Pacific countries, from established markets seeking technology upgrades to emerging economies investing in healthcare infrastructure development. The market’s future success will depend on companies’ ability to develop tailored solutions that address specific regional needs while maintaining clinical efficacy and economic value.
Strategic positioning for long-term success requires focus on innovation, clinical evidence development, and comprehensive market understanding. Companies that can effectively navigate regulatory requirements, build strong regional partnerships, and deliver integrated solutions addressing evolving healthcare needs are positioned to capture significant market opportunities in this expanding and dynamic market segment.
What is Cath Lab Hemodynamic Monitoring Systems?
Cath Lab Hemodynamic Monitoring Systems are specialized medical devices used to measure and monitor the hemodynamic parameters of patients undergoing cardiac procedures. These systems provide critical data on blood flow, pressure, and volume, which are essential for effective diagnosis and treatment in cath labs.
What are the key players in the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market?
Key players in the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market include companies like Philips Healthcare, GE Healthcare, and Siemens Healthineers, which are known for their innovative monitoring solutions and advanced technologies in the healthcare sector, among others.
What are the growth factors driving the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market?
The growth of the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market is driven by factors such as the increasing prevalence of cardiovascular diseases, advancements in medical technology, and the rising demand for minimally invasive procedures. Additionally, the growing aging population contributes to the market’s expansion.
What challenges does the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market face?
The Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market faces challenges such as high costs associated with advanced monitoring systems and the need for skilled professionals to operate these technologies. Furthermore, regulatory hurdles and varying healthcare infrastructure across countries can impede market growth.
What opportunities exist in the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market?
Opportunities in the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market include the potential for technological innovations, such as the integration of artificial intelligence and telemedicine solutions. Additionally, expanding healthcare facilities and increasing investments in healthcare infrastructure present significant growth prospects.
What trends are shaping the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market?
Trends shaping the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market include the shift towards patient-centric care, the adoption of remote monitoring technologies, and the increasing focus on data analytics for improved patient outcomes. These trends are driving the development of more sophisticated and user-friendly monitoring systems.
Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market
| Segmentation Details | Description |
|---|---|
| Product Type | Invasive Monitors, Non-Invasive Monitors, Wireless Monitors, Portable Monitors |
| Technology | Ultrasound, Electromagnetic, Optical, Pressure Sensors |
| End User | Hospitals, Cardiac Centers, Research Institutions, Emergency Departments |
| Application | Cardiac Surgery, Interventional Cardiology, Diagnostics, Patient Monitoring |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the Asia Pacific Cath Lab Hemodynamic Monitoring Systems Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at