444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at

Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$2750
The APAC infectious disease diagnosis and treatment market represents one of the most dynamic and rapidly evolving healthcare sectors in the Asia-Pacific region. This comprehensive market encompasses a wide range of diagnostic tools, therapeutic solutions, and preventive measures designed to combat various infectious diseases that continue to pose significant health challenges across diverse populations. The region’s unique demographic characteristics, varying healthcare infrastructure development levels, and emerging disease patterns create a complex yet promising landscape for infectious disease management.
Market dynamics in the APAC region are driven by several critical factors, including increasing disease prevalence, growing healthcare awareness, and substantial investments in medical infrastructure. The market demonstrates robust growth potential, with analysts projecting a compound annual growth rate (CAGR) of 8.2% over the forecast period. This growth trajectory reflects the region’s commitment to strengthening healthcare systems and improving patient outcomes through advanced diagnostic and treatment technologies.
Regional diversity plays a crucial role in shaping market characteristics, as countries like Japan, South Korea, and Australia maintain highly developed healthcare systems, while emerging economies such as India, Indonesia, and Vietnam are rapidly expanding their healthcare capabilities. This variation creates multiple market segments with distinct needs, regulatory requirements, and growth opportunities that companies must navigate strategically.
The APAC infectious disease diagnosis and treatment market refers to the comprehensive ecosystem of medical products, services, and technologies specifically designed to identify, monitor, and treat infectious diseases across the Asia-Pacific region. This market encompasses diagnostic equipment, laboratory testing services, pharmaceutical treatments, vaccines, and supportive care solutions that healthcare providers utilize to combat bacterial, viral, fungal, and parasitic infections affecting regional populations.
Diagnostic components include advanced molecular testing platforms, rapid diagnostic tests, immunoassays, and traditional culture-based methods that enable healthcare professionals to accurately identify pathogens and determine appropriate treatment protocols. The treatment segment encompasses antimicrobial medications, antiviral therapies, antifungal treatments, and supportive care products that help patients recover from infectious diseases while minimizing complications and preventing transmission.
Market scope extends beyond traditional healthcare settings to include point-of-care testing solutions, home-based diagnostic kits, telemedicine platforms, and digital health technologies that enhance accessibility and improve patient outcomes across diverse geographic and socioeconomic contexts throughout the Asia-Pacific region.
Strategic analysis of the APAC infectious disease diagnosis and treatment market reveals a sector characterized by significant growth potential, technological innovation, and evolving healthcare needs. The market benefits from increasing government healthcare investments, rising disease awareness, and growing demand for rapid, accurate diagnostic solutions that enable timely treatment interventions.
Key market drivers include the persistent threat of endemic diseases, emerging infectious disease outbreaks, and the ongoing need for antimicrobial resistance management. The COVID-19 pandemic has particularly highlighted the importance of robust diagnostic and treatment capabilities, leading to accelerated adoption of molecular testing technologies and increased investment in healthcare infrastructure across the region.
Competitive landscape features a mix of established multinational corporations, regional players, and emerging biotechnology companies that contribute to market innovation and accessibility. Major market participants focus on developing cost-effective solutions tailored to regional needs while maintaining high quality standards and regulatory compliance across diverse markets.
Growth projections indicate sustained market expansion driven by demographic trends, urbanization, climate change impacts on disease patterns, and increasing healthcare expenditure across APAC countries. The market demonstrates particular strength in molecular diagnostics, point-of-care testing, and personalized treatment approaches that align with regional healthcare priorities and patient needs.

Market intelligence reveals several critical insights that shape the APAC infectious disease diagnosis and treatment landscape. These insights provide valuable guidance for stakeholders seeking to understand market dynamics and identify strategic opportunities for growth and innovation.
Primary market drivers propelling growth in the APAC infectious disease diagnosis and treatment market stem from multiple interconnected factors that create sustained demand for innovative healthcare solutions. These drivers reflect both immediate healthcare needs and long-term strategic priorities across the region.
Disease burden factors represent the most significant driver, as the APAC region continues to face substantial challenges from both endemic and emerging infectious diseases. Tropical and subtropical climates in many countries create favorable conditions for vector-borne diseases, while dense urban populations facilitate rapid disease transmission. The persistent threat of tuberculosis, malaria, dengue fever, and other regional diseases maintains consistent demand for diagnostic and treatment solutions.
Healthcare infrastructure development across emerging economies drives market expansion as governments invest heavily in hospital construction, laboratory capabilities, and medical equipment procurement. Countries like India, Vietnam, and Indonesia are rapidly expanding their healthcare systems, creating new opportunities for diagnostic and treatment technology providers to establish market presence and build long-term partnerships.
Technological advancement in diagnostic capabilities enables more accurate, rapid, and cost-effective disease detection, driving adoption across healthcare settings. Innovations in molecular diagnostics, artificial intelligence-powered analysis, and portable testing devices make advanced diagnostic capabilities accessible in previously underserved markets, expanding the overall market reach and potential.
Regulatory support from government agencies encourages market development through favorable policies, streamlined approval processes, and financial incentives for healthcare innovation. Many APAC countries have implemented fast-track approval mechanisms for critical diagnostic and treatment technologies, particularly following lessons learned during the COVID-19 pandemic.
Market constraints in the APAC infectious disease diagnosis and treatment sector present significant challenges that companies must address to achieve sustainable growth and market penetration. These restraints vary considerably across different countries and market segments within the region.
Economic disparities across APAC countries create substantial barriers to market access, as healthcare budgets and patient purchasing power vary dramatically between developed and developing economies. While countries like Japan and Australia can afford premium diagnostic and treatment solutions, emerging markets often require more cost-effective alternatives that may compromise on advanced features or capabilities.
Regulatory complexity poses ongoing challenges as companies must navigate diverse regulatory frameworks, approval processes, and quality standards across multiple jurisdictions. Each country maintains distinct requirements for product registration, clinical trials, and market authorization, creating significant compliance costs and time delays for market entry.
Infrastructure limitations in rural and remote areas restrict market penetration for sophisticated diagnostic and treatment technologies that require reliable electricity, cold chain storage, or specialized technical support. These limitations particularly affect molecular diagnostic platforms and advanced therapeutic solutions that depend on consistent environmental conditions and skilled operators.
Skilled workforce shortages limit the effective deployment of advanced diagnostic and treatment technologies, as many regions lack sufficient numbers of trained laboratory technicians, healthcare professionals, and technical support personnel. This constraint affects both the adoption rate of new technologies and the quality of service delivery in healthcare settings.
Strategic opportunities in the APAC infectious disease diagnosis and treatment market present compelling prospects for companies willing to invest in innovation, partnerships, and market development initiatives. These opportunities align with regional healthcare priorities and emerging market trends that create favorable conditions for growth.
Digital health integration offers substantial opportunities as healthcare systems increasingly adopt telemedicine, remote monitoring, and digital diagnostic platforms. The COVID-19 pandemic accelerated digital health adoption across the region, creating new market segments for companies that can effectively combine traditional diagnostic and treatment capabilities with digital health technologies.
Point-of-care expansion represents a significant growth opportunity, particularly in rural and underserved areas where traditional laboratory infrastructure remains limited. Companies developing portable, user-friendly diagnostic devices and treatment solutions can capture substantial market share by addressing accessibility challenges that conventional healthcare delivery models cannot effectively resolve.
Antimicrobial stewardship programs create opportunities for companies offering rapid susceptibility testing, diagnostic decision support tools, and targeted therapeutic solutions. As antimicrobial resistance becomes an increasingly critical concern across the region, healthcare systems are investing in technologies that enable more precise, effective treatment protocols.
Public-private partnerships provide opportunities for companies to collaborate with government agencies on large-scale healthcare initiatives, infrastructure development projects, and disease surveillance programs. These partnerships can provide stable revenue streams while contributing to broader public health objectives across APAC countries.
Market dynamics in the APAC infectious disease diagnosis and treatment sector reflect complex interactions between technological innovation, healthcare policy changes, demographic trends, and economic development patterns. Understanding these dynamics is essential for stakeholders seeking to navigate market challenges and capitalize on emerging opportunities.
Supply chain evolution has become increasingly sophisticated as companies adapt to regional distribution challenges, regulatory requirements, and quality assurance standards. The pandemic highlighted the importance of resilient supply chains, leading to increased investment in regional manufacturing capabilities and strategic inventory management systems that can respond effectively to demand fluctuations and supply disruptions.
Competitive intensity continues to increase as new market entrants, including biotechnology startups and technology companies, challenge established players with innovative solutions and disruptive business models. This competition drives continuous innovation while creating pricing pressures that benefit healthcare systems and patients through improved access to advanced diagnostic and treatment technologies.
Customer behavior patterns are evolving as healthcare providers and patients become more sophisticated in their technology adoption decisions. There is growing demand for integrated solutions that combine diagnostic capabilities with treatment guidance, data management, and outcome tracking features that support comprehensive patient care and healthcare system efficiency.
Regulatory landscape changes across APAC countries are generally trending toward harmonization and streamlined approval processes, particularly for critical healthcare technologies. This evolution creates opportunities for companies to achieve faster market access while maintaining high quality and safety standards that protect patient welfare and support healthcare system confidence.
Comprehensive research methodology employed in analyzing the APAC infectious disease diagnosis and treatment market incorporates multiple data sources, analytical frameworks, and validation processes to ensure accuracy, reliability, and actionable insights for market stakeholders.
Primary research components include extensive interviews with healthcare professionals, laboratory managers, procurement specialists, and industry executives across major APAC markets. These interviews provide firsthand insights into market needs, technology preferences, purchasing decisions, and emerging trends that quantitative data alone cannot capture effectively.
Secondary research analysis encompasses comprehensive review of government health statistics, regulatory filings, company financial reports, clinical trial databases, and academic research publications. This analysis provides historical context, market sizing validation, and trend identification that supports primary research findings and market projections.
Market modeling techniques utilize advanced statistical methods, scenario analysis, and forecasting models that account for regional variations, economic factors, and healthcare policy changes. These models incorporate multiple variables to generate realistic market projections that reflect the complexity and diversity of APAC healthcare markets.
Data validation processes include cross-referencing multiple sources, expert review panels, and sensitivity analysis to ensure research findings accurately represent market conditions and provide reliable guidance for strategic decision-making. According to MarkWide Research analysis, this multi-layered validation approach enhances the credibility and utility of market intelligence for industry stakeholders.
Regional market analysis reveals significant variations in market characteristics, growth patterns, and strategic priorities across different APAC countries and sub-regions. These variations reflect diverse economic conditions, healthcare infrastructure development levels, disease patterns, and regulatory environments that shape market opportunities and challenges.
East Asian markets including Japan, South Korea, and China represent the most developed and largest segments of the APAC infectious disease diagnosis and treatment market. Japan maintains 23% of regional market share due to its advanced healthcare system, high technology adoption rates, and substantial healthcare spending. South Korea demonstrates strong growth in molecular diagnostics and digital health integration, while China’s vast population and rapidly expanding healthcare infrastructure create enormous market potential.
Southeast Asian countries including Indonesia, Thailand, Malaysia, and the Philippines show robust growth potential driven by economic development, healthcare infrastructure investment, and increasing disease awareness. These markets particularly favor cost-effective diagnostic solutions and point-of-care testing technologies that address accessibility challenges in diverse geographic and economic conditions.
South Asian markets led by India represent significant growth opportunities due to large populations, increasing healthcare investment, and growing private sector participation in healthcare delivery. India accounts for approximately 18% of regional market activity and demonstrates strong demand for affordable, scalable diagnostic and treatment solutions that can serve both urban and rural populations effectively.
Oceania markets including Australia and New Zealand maintain high technology adoption rates and sophisticated healthcare systems that drive demand for premium diagnostic and treatment solutions. These markets often serve as testing grounds for new technologies before broader APAC market introduction, making them strategically important for companies seeking regional market entry.
Competitive dynamics in the APAC infectious disease diagnosis and treatment market feature a diverse ecosystem of multinational corporations, regional specialists, and emerging technology companies that compete across multiple market segments and geographic regions.
Market positioning strategies vary significantly among competitors, with some focusing on premium technology solutions for developed markets while others emphasize cost-effective, accessible products for emerging economies. Companies increasingly pursue partnership strategies with local distributors, healthcare systems, and government agencies to enhance market penetration and service delivery capabilities.
Innovation competition drives continuous product development in areas such as rapid testing, artificial intelligence integration, and portable diagnostic platforms. Companies invest heavily in research and development to maintain competitive advantages while addressing evolving customer needs and regulatory requirements across diverse APAC markets.
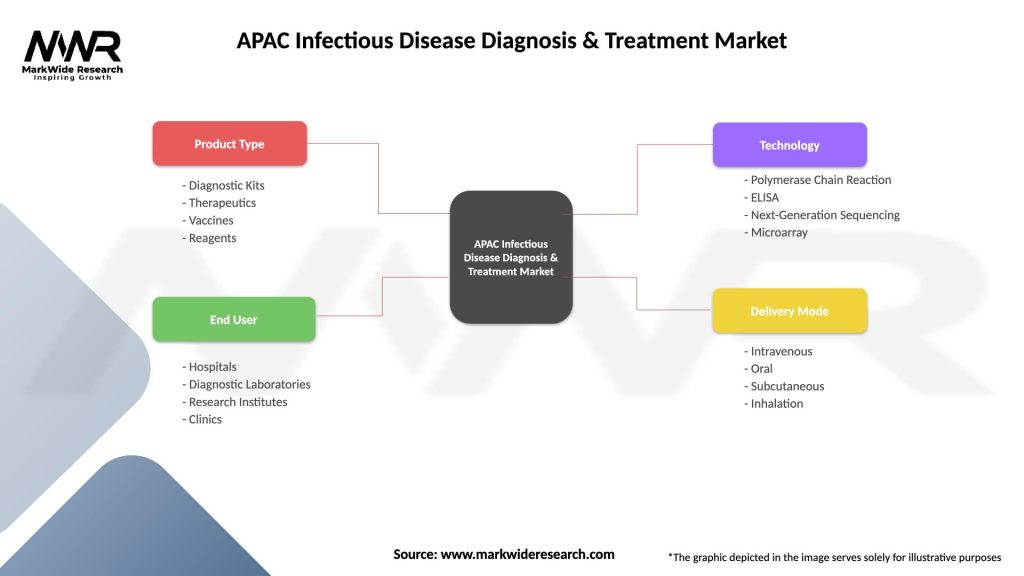
Market segmentation analysis provides detailed insights into the diverse components and applications that comprise the APAC infectious disease diagnosis and treatment market. This segmentation helps stakeholders understand market structure, identify growth opportunities, and develop targeted strategies for specific market segments.
By Product Type:
By Technology:
By Application:
Molecular diagnostics category represents the fastest-growing segment within the APAC infectious disease market, driven by increasing demand for rapid, accurate pathogen identification and antimicrobial resistance detection. This category benefits from technological advances that have reduced costs while improving sensitivity and specificity, making molecular testing more accessible across diverse healthcare settings.
Point-of-care testing segment demonstrates exceptional growth potential, particularly in rural and resource-limited settings where traditional laboratory infrastructure remains inadequate. The segment shows growth rates exceeding 12% annually as healthcare systems prioritize accessible, rapid diagnostic solutions that enable immediate treatment decisions and improve patient outcomes.
Immunodiagnostics applications maintain strong market presence due to their cost-effectiveness, ease of use, and broad applicability across various infectious diseases. This category particularly benefits from ongoing innovations in rapid test formats and digital result interpretation systems that enhance accuracy and reduce operator dependency.
Treatment solutions category encompasses antimicrobial medications, supportive care products, and therapeutic monitoring systems that complement diagnostic capabilities. This segment faces increasing pressure to address antimicrobial resistance challenges while maintaining therapeutic effectiveness across diverse patient populations and disease conditions.
Digital health integration across all categories creates new opportunities for enhanced diagnostic accuracy, treatment monitoring, and patient management. Companies that successfully integrate digital technologies with traditional diagnostic and treatment solutions achieve competitive advantages through improved customer value propositions and operational efficiency.
Healthcare providers benefit significantly from advanced infectious disease diagnosis and treatment solutions through improved patient outcomes, reduced treatment costs, and enhanced operational efficiency. Modern diagnostic technologies enable faster, more accurate pathogen identification, leading to targeted therapy selection and reduced antimicrobial resistance development.
Patients experience substantial benefits including faster diagnosis, more effective treatments, and reduced healthcare costs through improved diagnostic accuracy and targeted therapeutic interventions. Point-of-care testing solutions particularly benefit patients in remote areas by providing immediate access to diagnostic services without requiring travel to distant healthcare facilities.
Healthcare systems achieve multiple benefits including reduced disease transmission, improved resource allocation, and enhanced surveillance capabilities that support public health objectives. Advanced diagnostic and treatment technologies help healthcare systems respond more effectively to disease outbreaks while maintaining routine healthcare service quality.
Government agencies benefit from improved disease surveillance, better healthcare resource planning, and enhanced public health protection through comprehensive infectious disease management capabilities. These benefits support broader healthcare policy objectives while contributing to economic development through improved population health outcomes.
Industry participants gain access to expanding market opportunities, technological innovation partnerships, and sustainable revenue streams through participation in the growing APAC infectious disease diagnosis and treatment market. Companies that successfully address regional needs while maintaining quality standards achieve long-term competitive advantages and market leadership positions.
Strengths:
Weaknesses:
Opportunities:
Threats:
Technological convergence represents a dominant trend as companies integrate artificial intelligence, machine learning, and digital health platforms with traditional diagnostic and treatment solutions. This convergence enables more sophisticated analysis, predictive capabilities, and personalized treatment approaches that improve patient outcomes while reducing healthcare costs.
Point-of-care expansion continues accelerating as healthcare systems prioritize accessible, rapid diagnostic solutions that enable immediate treatment decisions. This trend particularly benefits rural and underserved populations while reducing healthcare system burden through decentralized testing capabilities and improved patient flow management.
Antimicrobial stewardship focus intensifies across the region as healthcare systems implement comprehensive programs to combat antimicrobial resistance. This trend drives demand for rapid susceptibility testing, diagnostic decision support tools, and targeted therapeutic solutions that optimize treatment effectiveness while minimizing resistance development.
Digital health integration accelerates as healthcare providers adopt telemedicine platforms, remote monitoring systems, and digital diagnostic tools that enhance accessibility and improve patient engagement. MWR data indicates that digital health adoption rates have increased by 67% across major APAC markets since the pandemic began.
Regulatory harmonization efforts across APAC countries aim to streamline approval processes and improve market access for innovative diagnostic and treatment technologies. This trend reduces compliance complexity while maintaining safety and efficacy standards that protect patient welfare and support healthcare system confidence.
Sustainability initiatives gain prominence as healthcare systems and companies focus on environmentally responsible practices, waste reduction, and sustainable supply chain management. This trend influences product design, packaging, and distribution strategies while supporting broader corporate social responsibility objectives.
Recent industry developments highlight the dynamic nature of the APAC infectious disease diagnosis and treatment market, with significant innovations, partnerships, and strategic initiatives shaping market evolution and competitive positioning.
Technology advancement initiatives include major investments in next-generation sequencing platforms, artificial intelligence-powered diagnostic systems, and portable molecular testing devices that enhance diagnostic capabilities while reducing costs and complexity. These developments particularly benefit resource-limited settings by making advanced diagnostic technologies more accessible and affordable.
Strategic partnership formations between multinational companies and regional healthcare organizations create new opportunities for market expansion, technology transfer, and service delivery improvement. These partnerships often focus on addressing specific regional needs while leveraging global expertise and resources to achieve sustainable market development.
Regulatory milestone achievements include streamlined approval processes for critical diagnostic and treatment technologies, particularly those addressing antimicrobial resistance and emerging infectious diseases. These achievements reduce time-to-market while maintaining safety and efficacy standards that support healthcare system confidence and patient protection.
Manufacturing capacity expansion across the region reflects growing demand and supply chain resilience priorities following pandemic-related disruptions. Companies are establishing regional production facilities to improve supply security while reducing costs and delivery times for APAC markets.
Digital health platform launches integrate diagnostic capabilities with telemedicine, remote monitoring, and data analytics solutions that enhance patient care while improving healthcare system efficiency. These platforms particularly benefit rural and underserved populations by providing access to specialized expertise and advanced diagnostic capabilities.
Strategic recommendations for companies operating in the APAC infectious disease diagnosis and treatment market emphasize the importance of regional adaptation, technology innovation, and partnership development to achieve sustainable competitive advantages and market success.
Market entry strategies should prioritize understanding local healthcare needs, regulatory requirements, and economic conditions before developing product portfolios and go-to-market approaches. Companies that invest time in market research and stakeholder engagement achieve better outcomes than those pursuing standardized global strategies without regional adaptation.
Technology development priorities should focus on solutions that address accessibility, affordability, and ease of use while maintaining high accuracy and reliability standards. Point-of-care testing, digital health integration, and antimicrobial stewardship applications represent particularly promising areas for innovation and market development.
Partnership strategies should emphasize collaboration with local distributors, healthcare systems, and government agencies to enhance market penetration and service delivery capabilities. These partnerships provide valuable market insights while supporting sustainable business development and customer relationship management.
Investment allocation should balance technology development with market development activities, ensuring that innovative solutions reach target customers effectively while generating sustainable returns. Companies should particularly focus on emerging markets where healthcare infrastructure development creates substantial growth opportunities.
Regulatory compliance strategies should anticipate evolving requirements while maintaining flexibility to adapt to changing standards and approval processes. Companies that establish strong regulatory capabilities early achieve faster market access and reduced compliance risks over time.
Long-term market prospects for the APAC infectious disease diagnosis and treatment market remain highly positive, driven by sustained healthcare investment, technological innovation, and growing disease awareness across the region. The market is expected to maintain robust growth rates as healthcare systems continue modernizing and expanding their capabilities to address evolving health challenges.
Technology evolution will likely focus on artificial intelligence integration, personalized medicine approaches, and enhanced connectivity that enables comprehensive patient care coordination. These advances will improve diagnostic accuracy while reducing costs and complexity, making advanced healthcare technologies accessible to broader populations across diverse economic conditions.
Market expansion patterns suggest continued growth in emerging economies as healthcare infrastructure development accelerates and economic conditions improve. Countries like Vietnam, Indonesia, and Bangladesh represent significant future opportunities as their healthcare systems mature and adopt advanced diagnostic and treatment technologies.
Regulatory landscape evolution toward greater harmonization and streamlined approval processes will likely reduce market entry barriers while maintaining safety and efficacy standards. This evolution supports innovation while protecting patient welfare and healthcare system integrity across APAC countries.
Sustainability considerations will increasingly influence product development, manufacturing, and distribution strategies as healthcare systems and companies prioritize environmental responsibility. This trend creates opportunities for companies that can develop effective solutions while minimizing environmental impact and supporting circular economy principles.
Growth projections indicate the market will continue expanding at healthy annual rates exceeding 8% through the forecast period, with particular strength in molecular diagnostics, point-of-care testing, and digital health integration segments that address regional healthcare priorities and patient needs effectively.
The APAC infectious disease diagnosis and treatment market represents a dynamic and rapidly evolving sector that offers substantial opportunities for companies willing to invest in innovation, regional adaptation, and strategic partnerships. Market growth is driven by increasing healthcare investment, technological advancement, and growing awareness of infectious disease challenges across diverse APAC countries.
Key success factors include understanding regional variations in healthcare needs, regulatory requirements, and economic conditions while developing solutions that address accessibility, affordability, and effectiveness priorities. Companies that successfully balance global expertise with local market knowledge achieve sustainable competitive advantages and long-term market leadership positions.
Future market development will likely emphasize digital health integration, antimicrobial stewardship, and point-of-care testing solutions that enhance healthcare accessibility while improving patient outcomes. According to MarkWide Research projections, these trends will continue driving market expansion and creating new opportunities for innovative companies and strategic partnerships.
The APAC infectious disease diagnosis and treatment market stands poised for continued growth and innovation, offering compelling opportunities for stakeholders committed to improving healthcare outcomes while building sustainable, profitable businesses across one of the world’s most dynamic and diverse healthcare markets.
What is Infectious Disease Diagnosis & Treatment?
Infectious Disease Diagnosis & Treatment refers to the methods and processes used to identify and manage infectious diseases. This includes diagnostic tests, treatment protocols, and preventive measures aimed at controlling the spread of infections.
What are the key players in the APAC Infectious Disease Diagnosis & Treatment Market?
Key players in the APAC Infectious Disease Diagnosis & Treatment Market include Roche Diagnostics, Abbott Laboratories, and Siemens Healthineers, among others. These companies are known for their innovative diagnostic solutions and treatment options for various infectious diseases.
What are the growth factors driving the APAC Infectious Disease Diagnosis & Treatment Market?
The growth of the APAC Infectious Disease Diagnosis & Treatment Market is driven by increasing incidences of infectious diseases, advancements in diagnostic technologies, and rising healthcare expenditure. Additionally, the growing awareness of infectious disease prevention contributes to market expansion.
What challenges does the APAC Infectious Disease Diagnosis & Treatment Market face?
The APAC Infectious Disease Diagnosis & Treatment Market faces challenges such as regulatory hurdles, high costs of advanced diagnostic equipment, and disparities in healthcare access across different regions. These factors can hinder the timely diagnosis and treatment of infectious diseases.
What opportunities exist in the APAC Infectious Disease Diagnosis & Treatment Market?
Opportunities in the APAC Infectious Disease Diagnosis & Treatment Market include the development of rapid diagnostic tests, increased investment in healthcare infrastructure, and the potential for telemedicine solutions. These advancements can enhance disease management and improve patient outcomes.
What trends are shaping the APAC Infectious Disease Diagnosis & Treatment Market?
Trends shaping the APAC Infectious Disease Diagnosis & Treatment Market include the integration of artificial intelligence in diagnostics, the rise of personalized medicine, and the focus on preventive healthcare. These trends are expected to transform how infectious diseases are diagnosed and treated.
APAC Infectious Disease Diagnosis & Treatment Market
| Segmentation Details | Description |
|---|---|
| Product Type | Diagnostic Kits, Therapeutics, Vaccines, Reagents |
| End User | Hospitals, Diagnostic Laboratories, Research Institutes, Clinics |
| Technology | Polymerase Chain Reaction, ELISA, Next-Generation Sequencing, Microarray |
| Delivery Mode | Intravenous, Oral, Subcutaneous, Inhalation |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading companies in the APAC Infectious Disease Diagnosis & Treatment Market
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at