444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Cannabinoid Intoxication Treatment Drug market is witnessing substantial growth, propelled by the increasing prevalence of cannabinoid intoxication cases, rising legalization of cannabis, and the need for effective treatment options for acute cannabis-related symptoms. Cannabinoid intoxication, often characterized by symptoms such as impaired cognition, anxiety, paranoia, and hallucinations, presents challenges for healthcare providers seeking to manage acute intoxication episodes and prevent adverse outcomes. Treatment drugs targeting cannabinoid receptors and modulating neurotransmitter activity offer potential therapeutic benefits in alleviating acute intoxication symptoms and reducing the risk of complications associated with cannabis use.
Meaning
Cannabinoid intoxication treatment drugs are pharmaceutical agents designed to counteract the effects of excessive cannabis consumption by modulating the activity of cannabinoid receptors in the brain and central nervous system. These drugs may act as agonists, antagonists, or allosteric modulators of cannabinoid receptors, influencing neurotransmitter release, synaptic transmission, and neuronal excitability to mitigate acute intoxication symptoms and restore cognitive function. Cannabinoid intoxication treatment drugs may be administered orally, intravenously, or via other routes depending on the severity of symptoms and the clinical setting.
Executive Summary
The Cannabinoid Intoxication Treatment Drug market is experiencing rapid expansion, driven by factors such as the increasing availability of potent cannabis products, changes in cannabis legislation, and the growing recognition of cannabinoid hyperemesis syndrome (CHS) and other cannabis-related health complications. Key players in the market are focusing on developing novel treatment drugs with improved efficacy, safety, and tolerability profiles, addressing unmet medical needs in acute intoxication management and substance abuse treatment. With the evolving landscape of cannabis use and legalization, the market for cannabinoid intoxication treatment drugs is poised for continued growth and innovation.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Cannabinoid Intoxication Treatment Drug market is characterized by dynamic trends and evolving regulatory landscapes influenced by factors such as changing patterns of cannabis use, public health priorities, and scientific advancements in cannabinoid pharmacology and therapeutics. Understanding these dynamics is essential for stakeholders to navigate the complex landscape of cannabis-related healthcare and capitalize on emerging opportunities in cannabinoid intoxication treatment and prevention.
Regional Analysis
North America dominates the global Cannabinoid Intoxication Treatment Drug market, driven by factors such as the widespread legalization of recreational cannabis in certain states, the growing demand for substance abuse treatment services, and the increasing recognition of cannabinoid hyperemesis syndrome (CHS) as a clinical entity requiring medical intervention. Europe and other regions are also significant markets, with varying regulatory approaches to cannabis legalization, medical cannabis access, and public health interventions aimed at mitigating cannabis-related harms.
Competitive Landscape
Leading Companies in Cannabinoid Intoxication Treatment Drug Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
Segmentation
The Cannabinoid Intoxication Treatment Drug market can be segmented based on drug class, mechanism of action, route of administration, and therapeutic indication. Drug classes may include cannabinoid receptor agonists, antagonists, partial agonists, allosteric modulators, and enzyme inhibitors targeting the endocannabinoid system. Mechanisms of action may involve modulation of CB1 receptors, CB2 receptors, or other components of the endocannabinoid signaling pathway. Routes of administration may include oral, sublingual, intravenous, or inhalational delivery depending on the pharmacokinetic properties and therapeutic indications of specific drug formulations.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The COVID-19 pandemic has highlighted the importance of cannabis-related health surveillance, public health interventions, and treatment access for individuals with cannabis use disorders and cannabis-related medical emergencies. While the pandemic has disrupted healthcare services and substance abuse treatment programs, it has also underscored the need for innovative approaches to cannabis harm reduction, telemedicine-based interventions, and remote patient monitoring in the context of social distancing measures and healthcare resource constraints.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Cannabinoid Intoxication Treatment Drug market is poised for significant growth and innovation, driven by factors such as changing patterns of cannabis use, evolving regulatory landscapes, and advancements in cannabinoid pharmacology and therapeutics. With the increasing recognition of cannabinoid intoxication as a clinical entity requiring medical intervention, there are opportunities for stakeholders to develop safe, effective, and evidence-based treatment options for managing acute intoxication symptoms, preventing cannabis-related health complications, and supporting individuals with cannabis use disorders on their path to recovery and wellness.
Conclusion
In conclusion, the Cannabinoid Intoxication Treatment Drug market presents promising opportunities for pharmaceutical companies, researchers, healthcare providers, and policymakers to address the growing public health concerns associated with cannabis use and cannabinoid intoxication. By investing in research, education, and collaborative efforts, stakeholders can advance the development of novel treatment drugs, evidence-based interventions, and comprehensive strategies for managing acute intoxication symptoms, reducing cannabis-related harms, and promoting the health and well-being of individuals affected by cannabis use disorders and cannabis-related health conditions.
What is Cannabinoid Intoxication Treatment Drug?
Cannabinoid Intoxication Treatment Drug refers to medications specifically designed to alleviate the effects of cannabinoid intoxication, which can occur from the consumption of cannabis products. These treatments aim to manage symptoms such as anxiety, confusion, and impaired motor function.
What are the key players in the Cannabinoid Intoxication Treatment Drug Market?
Key players in the Cannabinoid Intoxication Treatment Drug Market include companies like GW Pharmaceuticals, Canopy Growth Corporation, and Tilray, among others. These companies are involved in the development and distribution of cannabinoid-based therapies and treatments.
What are the growth factors driving the Cannabinoid Intoxication Treatment Drug Market?
The Cannabinoid Intoxication Treatment Drug Market is driven by increasing cannabis legalization, rising awareness of cannabinoid effects, and a growing number of patients seeking treatment for cannabinoid-related issues. Additionally, advancements in research and development are contributing to market growth.
What challenges does the Cannabinoid Intoxication Treatment Drug Market face?
Challenges in the Cannabinoid Intoxication Treatment Drug Market include regulatory hurdles, varying legal statuses of cannabis across regions, and potential stigma associated with cannabinoid use. These factors can hinder market expansion and product acceptance.
What opportunities exist in the Cannabinoid Intoxication Treatment Drug Market?
Opportunities in the Cannabinoid Intoxication Treatment Drug Market include the potential for new product development targeting specific symptoms of intoxication and the expansion into emerging markets where cannabis use is increasing. Additionally, partnerships with healthcare providers can enhance treatment accessibility.
What trends are shaping the Cannabinoid Intoxication Treatment Drug Market?
Trends in the Cannabinoid Intoxication Treatment Drug Market include the rise of personalized medicine approaches, increased research into cannabinoid pharmacology, and the development of non-psychoactive treatment options. These trends are influencing how treatments are formulated and delivered.
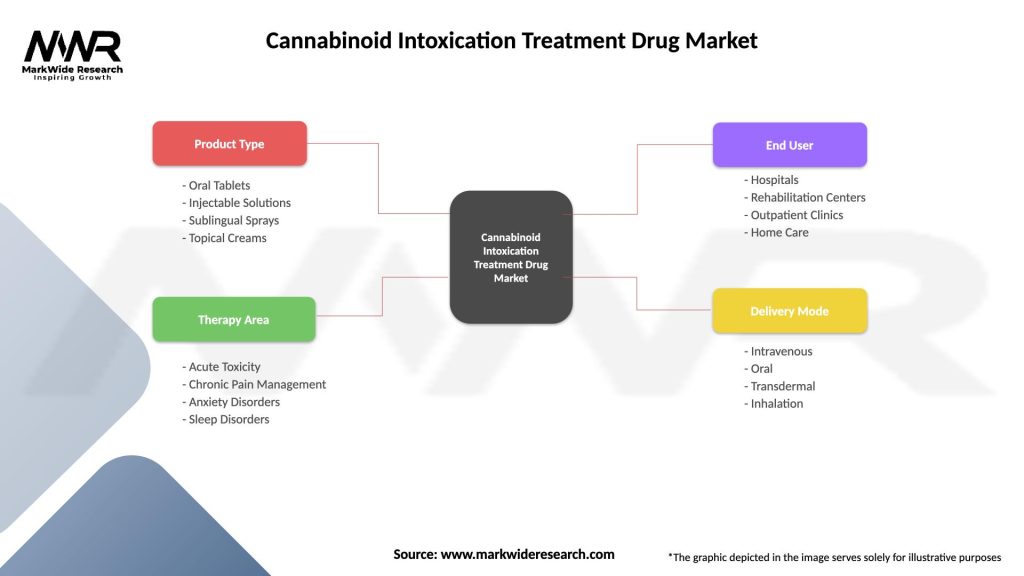
Cannabinoid Intoxication Treatment Drug Market
| Segmentation Details | Description |
|---|---|
| Product Type | Oral Tablets, Injectable Solutions, Sublingual Sprays, Topical Creams |
| Therapy Area | Acute Toxicity, Chronic Pain Management, Anxiety Disorders, Sleep Disorders |
| End User | Hospitals, Rehabilitation Centers, Outpatient Clinics, Home Care |
| Delivery Mode | Intravenous, Oral, Transdermal, Inhalation |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at