444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview:
The Hemostatic Valves and Accessories Market encompasses a range of medical devices designed to control bleeding and maintain vascular integrity during diagnostic and interventional procedures. These devices play a critical role in ensuring hemostasis and preventing complications in patients undergoing minimally invasive vascular interventions, such as angiography, angioplasty, stent placement, and cardiac catheterization.
Meaning:
Hemostatic valves and accessories are specialized medical devices used in conjunction with catheters, guidewires, and other interventional tools to facilitate access to blood vessels, deliver therapeutic agents, and manage hemostasis during vascular procedures. These devices feature one-way valve mechanisms, hemostatic seals, and ergonomic designs to minimize blood loss, reduce the risk of air embolism, and optimize procedural efficiency and safety.
Executive Summary:
The Hemostatic Valves and Accessories Market has experienced significant growth due to the increasing prevalence of cardiovascular diseases, rising demand for minimally invasive procedures, and technological advancements in vascular access and closure techniques. These devices offer healthcare providers enhanced procedural control, reduced complication rates, and improved patient outcomes. However, challenges such as regulatory compliance, product standardization, and reimbursement limitations impact market growth and innovation.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights:
Market Drivers:
Market Restraints:
Market Opportunities:
Market Dynamics:
The Hemostatic Valves and Accessories Market operates within a dynamic healthcare ecosystem influenced by technological innovation, regulatory reforms, market competition, and patient-driven demand for advanced vascular closure solutions. Adapting to market dynamics requires agility, innovation, collaboration, and strategic foresight from industry participants across the value chain.
Regional Analysis:
Regional variations in healthcare infrastructure, regulatory frameworks, reimbursement policies, and patient demographics impact market dynamics, product adoption rates, and healthcare provider preferences for hemostatic valves and accessory technologies. Tailoring market strategies and product offerings to regional needs and preferences optimizes market penetration and patient access to innovative vascular closure solutions.
Competitive Landscape:
Leading Companies in the Hemostatic Valves and Accessories Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
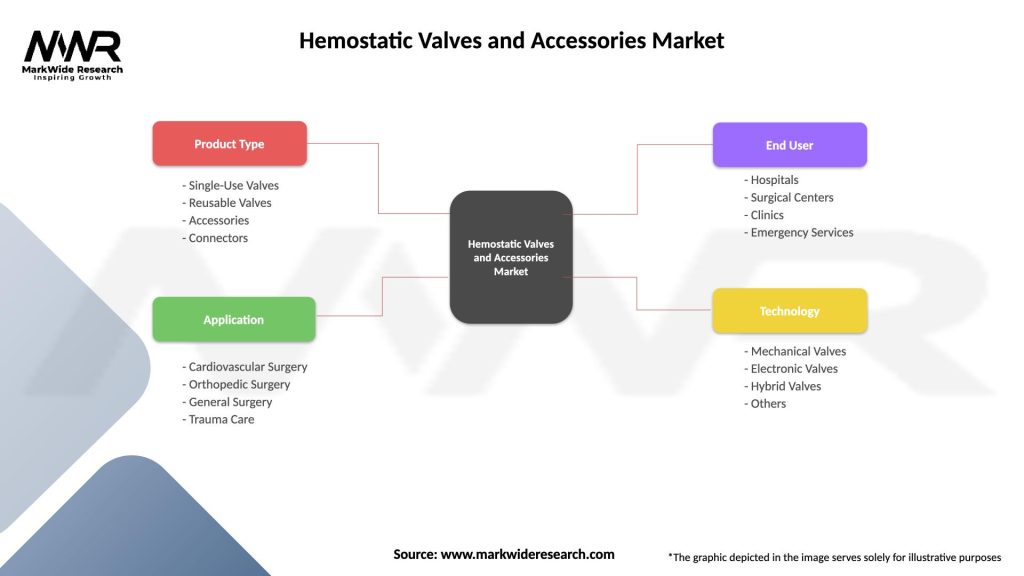
Segmentation:
Segmentation of the Hemostatic Valves and Accessories Market based on product type, application, end-user, and geographical region provides insights into market dynamics, patient needs, and growth opportunities, guiding product development, marketing strategies, and market expansion efforts.
Category-wise Insights:
Insights into different categories of hemostatic valves and accessory products, including vascular closure devices, hemostatic patches, compression devices, and closure sealants, offer a comprehensive understanding of product features, functionalities, indications, and patient preferences, facilitating informed decision-making and product selection by healthcare providers and patients.
Key Benefits for Industry Participants and Stakeholders:
Industry participants and stakeholders in the Hemostatic Valves and Accessories Market benefit from:
SWOT Analysis:
A SWOT analysis of the Hemostatic Valves and Accessories Market provides insights into its strengths, weaknesses, opportunities, and threats:
Understanding these factors enables industry participants to capitalize on market strengths, address weaknesses, leverage opportunities, and mitigate threats, optimizing their market position and strategic decision-making.
Market Key Trends:
Covid-19 Impact:
The Covid-19 pandemic has influenced the Hemostatic Valves and Accessories Market by:
Key Industry Developments:
Analyst Suggestions:
Future Outlook:
The future outlook for the Hemostatic Valves and Accessories Market is promising, with continued growth driven by technological innovation, clinical validation, regulatory compliance, and market expansion initiatives. Market opportunities abound in emerging applications, remote monitoring solutions, telemedicine integration, and global market expansion strategies, positioning hemostatic valve and accessory manufacturers for sustained success and market leadership in the years to come.
Conclusion:
The Hemostatic Valves and Accessories Market represents a dynamic and rapidly evolving healthcare segment characterized by technological innovation, clinical validation, regulatory compliance, and market expansion initiatives. Despite challenges posed by regulatory complexities, product standardization, and reimbursement limitations, industry participants are well-positioned to capitalize on market opportunities through investment in R&D, regulatory compliance, clinical validation, and strategic partnerships. As the market continues to evolve, strategic investments in technology, clinical research, regulatory affairs, and market expansion will be crucial for maintaining competitiveness, driving innovation, and meeting the evolving needs of healthcare providers and patients worldwide.
What is Hemostatic Valves and Accessories?
Hemostatic valves and accessories are medical devices used to control bleeding during surgical procedures. They are designed to maintain hemostasis by preventing blood loss while allowing the passage of instruments or fluids.
What are the key players in the Hemostatic Valves and Accessories Market?
Key players in the Hemostatic Valves and Accessories Market include companies like Medtronic, Boston Scientific, and Abbott Laboratories, which are known for their innovative medical devices and technologies, among others.
What are the growth factors driving the Hemostatic Valves and Accessories Market?
The growth of the Hemostatic Valves and Accessories Market is driven by the increasing number of surgical procedures, advancements in medical technology, and a rising focus on patient safety and effective blood management.
What challenges does the Hemostatic Valves and Accessories Market face?
Challenges in the Hemostatic Valves and Accessories Market include stringent regulatory requirements, high costs of advanced devices, and the need for continuous innovation to meet evolving surgical demands.
What opportunities exist in the Hemostatic Valves and Accessories Market?
Opportunities in the Hemostatic Valves and Accessories Market include the development of new materials and technologies, expansion into emerging markets, and increasing demand for minimally invasive surgical procedures.
What trends are shaping the Hemostatic Valves and Accessories Market?
Trends in the Hemostatic Valves and Accessories Market include the integration of smart technologies in surgical devices, a growing emphasis on personalized medicine, and the rise of robotic-assisted surgeries.
Hemostatic Valves and Accessories Market
| Segmentation Details | Description |
|---|---|
| Product Type | Single-Use Valves, Reusable Valves, Accessories, Connectors |
| Application | Cardiovascular Surgery, Orthopedic Surgery, General Surgery, Trauma Care |
| End User | Hospitals, Surgical Centers, Clinics, Emergency Services |
| Technology | Mechanical Valves, Electronic Valves, Hybrid Valves, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Hemostatic Valves and Accessories Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at