444 Alaska Avenue
Suite #BAA205 Torrance, CA 90503 USA
+1 424 999 9627
24/7 Customer Support
sales@markwideresearch.com
Email us at
Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at
Corporate User License
Unlimited User Access, Post-Sale Support, Free Updates, Reports in English & Major Languages, and more
$3450
Market Overview
The Activated Protein C Market is experiencing significant growth driven by the increasing demand for therapeutic agents targeting coagulation disorders and inflammatory conditions. Activated protein C (APC) is a serine protease that plays a critical role in regulating blood coagulation, inflammation, and endothelial function. With its antithrombotic, anti-inflammatory, and cytoprotective properties, APC has emerged as a promising therapeutic agent for the treatment of severe sepsis, disseminated intravascular coagulation (DIC), and other thrombotic disorders. As the prevalence of these conditions continues to rise, fueled by factors such as aging population, chronic diseases, and hospital-acquired infections, the market for activated protein C presents opportunities for pharmaceutical companies to develop novel therapies, expand treatment options, and improve patient outcomes.
Meaning
Activated protein C (APC) is a natural anticoagulant protein produced in the body that plays a crucial role in regulating blood coagulation and inflammation. It is synthesized in the endothelium and circulates in the bloodstream in an inactive form. Upon activation by thrombin and binding to its cofactor, protein S, APC exerts its anticoagulant effects by inhibiting factors Va and VIIIa, thereby preventing the formation of thrombin and the propagation of the coagulation cascade. In addition to its anticoagulant activity, APC has anti-inflammatory and cytoprotective properties, including the modulation of endothelial cell function, suppression of cytokine production, and inhibition of leukocyte adhesion and migration. These multifaceted effects make APC an attractive therapeutic target for the management of conditions characterized by dysregulated coagulation and inflammation, such as severe sepsis, DIC, and acute respiratory distress syndrome (ARDS).
Executive Summary
The Activated Protein C Market is witnessing robust growth driven by the increasing recognition of APC’s therapeutic potential in treating coagulation disorders and inflammatory conditions. APC offers a unique mechanism of action that targets multiple pathways involved in thrombosis, inflammation, and endothelial dysfunction, making it a promising candidate for the treatment of life-threatening conditions such as severe sepsis and DIC. With ongoing research efforts, clinical trials, and regulatory approvals, pharmaceutical companies are actively developing APC-based therapies to address unmet medical needs and improve patient outcomes. This presents opportunities for innovation, collaboration, and market expansion in the field of thrombosis and hemostasis.
Important Note: The companies listed in the image above are for reference only. The final study will cover 18–20 key players in this market, and the list can be adjusted based on our client’s requirements.
Key Market Insights
Market Drivers
Market Restraints
Market Opportunities
Market Dynamics
The Activated Protein C Market is characterized by dynamic trends and evolving market dynamics driven by factors such as disease prevalence, therapeutic innovation, regulatory changes, and healthcare economics. Key drivers of market growth include the increasing incidence of coagulation disorders, growing awareness of APC’s therapeutic benefits, and advancements in biotechnology and drug delivery systems. Market expansion is further fueled by the development of novel APC formulations, optimization of dosing regimens, and exploration of combination therapies targeting complementary pathways in thrombosis and inflammation. However, challenges such as production complexities, regulatory uncertainties, and safety concerns pose constraints to market growth, requiring stakeholders to collaborate and innovate to overcome these obstacles.
Regional Analysis
The Activated Protein C Market exhibits regional variations, with developed economies such as North America, Europe, and Asia-Pacific leading in terms of market size, research infrastructure, and healthcare expenditures. However, emerging economies in Latin America, Middle East, and Africa offer significant growth opportunities due to the increasing prevalence of coagulation disorders, rising healthcare investments, and government initiatives aimed at improving access to critical care services. Market expansion in these regions is further facilitated by the presence of a large patient population, growing awareness of APC’s therapeutic benefits, and regulatory reforms supporting drug development and commercialization.
Competitive Landscape
Leading Companies in the Activated Protein C Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
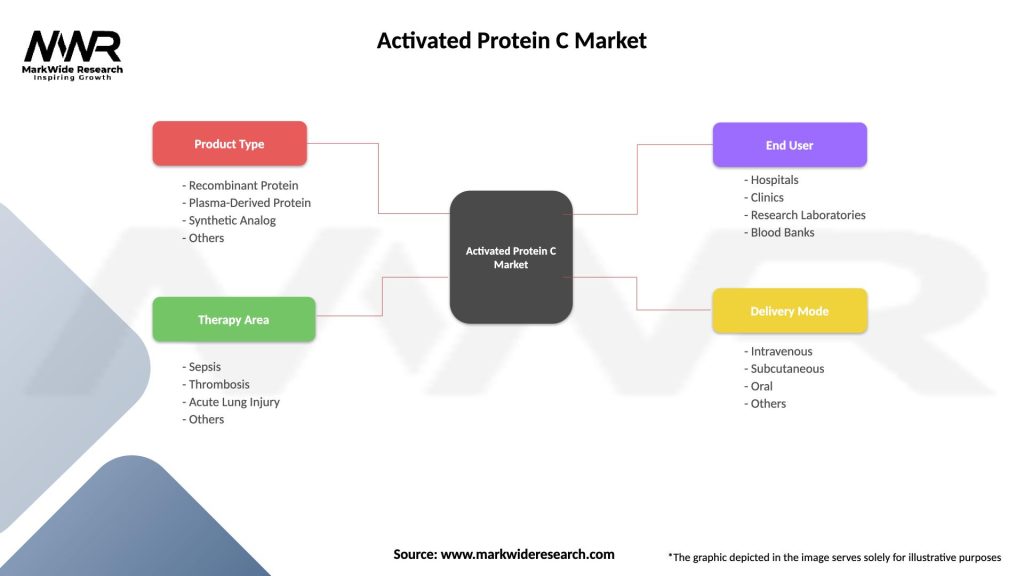
Segmentation
The Activated Protein C Market can be segmented based on product type, indication, administration route, and geography. Product types include recombinant APC (rAPC), plasma-derived APC, and APC analogs, reflecting different sources and formulations of APC-based therapies. Indications encompass severe sepsis, DIC, ARDS, ischemic stroke, and other thrombotic disorders characterized by dysregulated coagulation and inflammation. Administration routes include intravenous infusion, subcutaneous injection, and inhalation therapy, addressing diverse patient populations and clinical settings. Geographical segments include North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa, reflecting regional variations in disease prevalence, healthcare infrastructure, and regulatory environments.
Category-wise Insights
Key Benefits for Industry Participants and Stakeholders
SWOT Analysis
Market Key Trends
Covid-19 Impact
The Covid-19 pandemic has had a mixed impact on the Activated Protein C Market, with disruptions to healthcare systems, clinical trials, and supply chains worldwide. While the pandemic has highlighted the importance of APC’s anticoagulant and anti-inflammatory properties in managing severe sepsis and thrombotic complications in Covid-19 patients, it has also posed challenges such as delays in clinical research, regulatory approvals, and patient access to APC-based therapies. As healthcare providers adapt to the challenges of the pandemic and prioritize the treatment of Covid-19-related complications, there is a growing recognition of APC’s therapeutic potential in improving patient outcomes and reducing morbidity and mortality associated with thrombotic disorders and systemic inflammation.
Key Industry Developments
Analyst Suggestions
Future Outlook
The Activated Protein C Market is poised for continued growth and innovation, driven by factors such as therapeutic innovation, expanding indications, and increasing demand for novel therapies targeting coagulation disorders and inflammatory conditions. With ongoing investment in research and development, clinical evidence generation, and regulatory approvals, pharmaceutical companies are well-positioned to capitalize on emerging trends, meet evolving patient needs, and drive advancements in thrombosis and hemostasis. By leveraging technology, collaboration, and education, stakeholders in the Activated Protein C Market can contribute to improved patient outcomes, reduced healthcare costs, and enhanced quality of life for individuals worldwide.
Conclusion
In conclusion, the Activated Protein C Market represents a dynamic and promising segment of the pharmaceutical industry, offering innovative therapies for the management of coagulation disorders and inflammatory conditions. With its multifaceted mechanism of action, therapeutic versatility, and clinical evidence supporting its efficacy and safety, activated protein C has emerged as a valuable therapeutic agent for treating severe sepsis, DIC, and other thrombotic disorders. By investing in research and development, regulatory compliance, and market expansion, pharmaceutical companies, healthcare providers, and regulatory agencies can address unmet medical needs, improve patient outcomes, and drive progress in thrombosis and hemostasis.
What is Activated Protein C?
Activated Protein C is a protein in the blood that plays a crucial role in regulating blood coagulation and inflammation. It is involved in the proteolytic cleavage of specific coagulation factors, which helps to maintain hemostasis and prevent excessive clotting.
What are the key players in the Activated Protein C Market?
Key players in the Activated Protein C Market include companies such as Baxter International, Novo Nordisk, and Boehringer Ingelheim, which are known for their contributions to the development and commercialization of activated protein C therapies, among others.
What are the growth factors driving the Activated Protein C Market?
The Activated Protein C Market is driven by factors such as the increasing prevalence of thromboembolic disorders, advancements in biotechnology, and the growing awareness of the therapeutic benefits of activated protein C in critical care settings.
What challenges does the Activated Protein C Market face?
Challenges in the Activated Protein C Market include regulatory hurdles, high development costs, and competition from alternative therapies that may limit market growth and adoption.
What opportunities exist in the Activated Protein C Market?
Opportunities in the Activated Protein C Market include the potential for new product development, expanding applications in various therapeutic areas, and increasing investment in research and development to enhance treatment options.
What trends are shaping the Activated Protein C Market?
Trends in the Activated Protein C Market include a focus on personalized medicine, the integration of advanced technologies in drug delivery systems, and ongoing research into the broader applications of activated protein C in various medical conditions.
Activated Protein C Market
| Segmentation Details | Description |
|---|---|
| Product Type | Recombinant Protein, Plasma-Derived Protein, Synthetic Analog, Others |
| Therapy Area | Sepsis, Thrombosis, Acute Lung Injury, Others |
| End User | Hospitals, Clinics, Research Laboratories, Blood Banks |
| Delivery Mode | Intravenous, Subcutaneous, Oral, Others |
Please note: The segmentation can be entirely customized to align with our client’s needs.
Leading Companies in the Activated Protein C Market:
Please note: This is a preliminary list; the final study will feature 18–20 leading companies in this market. The selection of companies in the final report can be customized based on our client’s specific requirements.
North America
o US
o Canada
o Mexico
Europe
o Germany
o Italy
o France
o UK
o Spain
o Denmark
o Sweden
o Austria
o Belgium
o Finland
o Turkey
o Poland
o Russia
o Greece
o Switzerland
o Netherlands
o Norway
o Portugal
o Rest of Europe
Asia Pacific
o China
o Japan
o India
o South Korea
o Indonesia
o Malaysia
o Kazakhstan
o Taiwan
o Vietnam
o Thailand
o Philippines
o Singapore
o Australia
o New Zealand
o Rest of Asia Pacific
South America
o Brazil
o Argentina
o Colombia
o Chile
o Peru
o Rest of South America
The Middle East & Africa
o Saudi Arabia
o UAE
o Qatar
o South Africa
o Israel
o Kuwait
o Oman
o North Africa
o West Africa
o Rest of MEA
Trusted by Global Leaders
Fortune 500 companies, SMEs, and top institutions rely on MWR’s insights to make informed decisions and drive growth.
ISO & IAF Certified
Our certifications reflect a commitment to accuracy, reliability, and high-quality market intelligence trusted worldwide.
Customized Insights
Every report is tailored to your business, offering actionable recommendations to boost growth and competitiveness.
Multi-Language Support
Final reports are delivered in English and major global languages including French, German, Spanish, Italian, Portuguese, Chinese, Japanese, Korean, Arabic, Russian, and more.
Unlimited User Access
Corporate License offers unrestricted access for your entire organization at no extra cost.
Free Company Inclusion
We add 3–4 extra companies of your choice for more relevant competitive analysis — free of charge.
Post-Sale Assistance
Dedicated account managers provide unlimited support, handling queries and customization even after delivery.
GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


GET A FREE SAMPLE REPORT
This free sample study provides a complete overview of the report, including executive summary, market segments, competitive analysis, country level analysis and more.
ISO AND IAF CERTIFIED


Suite #BAA205 Torrance, CA 90503 USA
24/7 Customer Support
Email us at